Historically, the first descriptions of atherosclerotic lesions underlying acute coronary syndromes (ACS) were provided by post-mortem studies.1–3 Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) enable in vivo visualization of culprit lesions for ACS. IVUS and OCT generate high-resolution images of the coronary artery that help delineate the pathogenesis of atherosclerosis, specifically the major contributors for atherothrombotic events leading to unstable angina, MI, and sudden cardiac death. This review summarizes the data on the common atherothrombotic and non-atherothrombotic causes of ACS. We discuss how intravascular imaging may be used to optimize the outcomes of percutaneous coronary intervention (PCI) in ACS.
Plaque Rupture, Plaque Erosion, and Calcified Nodules: Histopathological Insights
Atherosclerotic plaques with a disrupted fibrous cap (ruptured or eroded) and eruptive calcified nodules can lead to ACS. In a large post-mortem study of 442 individuals with sudden cardiac death, acute thrombotic events were responsible for 234 (53%) of the deaths.4 Three culprit phenotypes accounted for most cases: ruptured fibrous caps (RFCs; 35%), intact fibrous caps (IFCs; 35%), and calcified nodules (5%).4 The pathophysiology of these three substrates of ACS is briefly reviewed below.
Pathophysiology of Ruptured Fibrous Caps
Fatty streaks are the initial atherosclerotic substrate. They are made up of foam cells that are deposited beneath the endothelium, and eventually progress to fibroatheromas. Thin cap fibroatheromas (TCFAs) can rupture as a result of biomechanical forces that are generated by shear stress and turbulent blood flow.5,6 In addition to the biomechanical stress, inflammatory processes, driven primarily by activated macrophages, that release proteases can degrade the thin cap and contribute to the rupture of the thin fibrous cap.7 The most vulnerable region in the fibrous cap is the shoulder region, which is where the normal tissue meets the atherosclerotic plaque.7 Ruptures usually occur in the shoulder region at rest and either in the shoulder region or the mid-fibrous cap during exercise.8
Microcalcification (~10 μm in size) is generated by necrotic macrophages or smooth muscle cells. Microcalcification is thought to make plaques vulnerable to rupture under high radial strain because it can act as a nidus for debonding of the fibrous cap.6,9,10 Rupture of the fibrous cap leads to exposure of the necrotic core formed within the plaque to the intravascular milieu. The necrotic core is highly thrombogenic and its exposure leads to the release of tissue factor, which acts as an intermediary for thrombus formation.7,11 This cascade of events leads to the formation of the red thrombus superimposed on RFCs, which is primarily composed of fibrin and red blood cells.12
Pathophysiology of Intact Fibrous Caps
In IFCs there is a communication between the luminal thrombus and intimal wall (with proteoglycans acting as the tissue–thrombus interface) that may lead to coronary obstruction.7,13 IFCs are non-ruptured fibroatheromas that are mostly composed of type III collagen; in comparison, RFCs mostly contain type I collagen.14 Plaques with IFCs can cause ACS in healthy young females and smokers.15
In addition to collagen composition, IFCs and RFCs can be distinguished by the distinct patterns of vascular remodeling they are associated with. Negative remodeling is defined as a decrease in the vessel diameter distal to the culprit lesion of ACS, whereas positive remodeling is an increase in the diameter of the vessel. IFCs are mostly associated with negative remodeling and RFCs are associated with positive remodeling.16 On histopathological examination, downstream embolization of thrombi to the microvasculature of the coronary arteries in IFC-ACS is a common finding, and IFCs are more likely to cause embolization than RFCs (71% versus 42%; p=0.01).13
Pathophysiology of Calcified Nodules
On histology, eruptive calcified nodules appear as microthrombi around a nodule that is protruding through the plaque surface, and calcified nodules are the least common atherothrombotic cause of ACS.4 The exact pathogenic mechanism of calcified nodules is not known, but activated macrophages can deposit large amounts of calcium, which can eventually fracture due to cyclic torsion of the artery to form a calcific protruding nodule.12 This calcification is distinct from the microcalcification observed in RFCs and IFCs and is usually seen in the vasculature with high flexion stress, such as in the mid-segments of the right coronary or left anterior descending arteries.17
Turbulent flow creates small nodules surrounded by fibrin on the surface of the nodule and, over time, these nodules grow through the fibrin meshwork, forming an eruptive calcified nodule.12 Eccentric calcification and eruptive calcified nodules disrupt the coronary endothelium, activating platelets in the epicardial coronary vessels, especially in older men.3
Intravascular Imaging in ACS
Angioscopy, IVUS, near-infrared spectroscopy, and near-infrared fluorescence may be used for imaging RFCs; however, OCT can most reliably identify culprit plaques with RFC.18 OCT uses near-infrared light to penetrate the vessel wall, producing cross-sectional images while blood is flushed with contrast. The infrared light in OCT has a wavelength of 1.3 μm, whereas the wavelength of the ultrasound wave in IVUS is 40 μm; therefore, IVUS provides lower resolution than OCT.19 Each modality is unique in its ability to visualize coronary lesions with different characteristics.
OCT provides detailed definition of the superficial structures because the depth of light penetration into the vessel wall is 1–2 mm, whereas IVUS can image deeper structures because the penetration depth of ultrasound waves is up to 6 mm. Thus, OCT and IVUS can be complementary, and hybrid imaging using both modalities in one catheter has attracted attention.19 Based on a total of 17 studies in 6,244 patients presenting with ACS (Supplementary Material Table 1), OCT revealed that 3,521 patients had plaques with RFC, 2,203 had plaques with IFC, and 316 had calcified nodules. Overall, these data are consistent with post-mortem histopathological studies.
Plaques with Ruptured Fibrous Caps on OCT
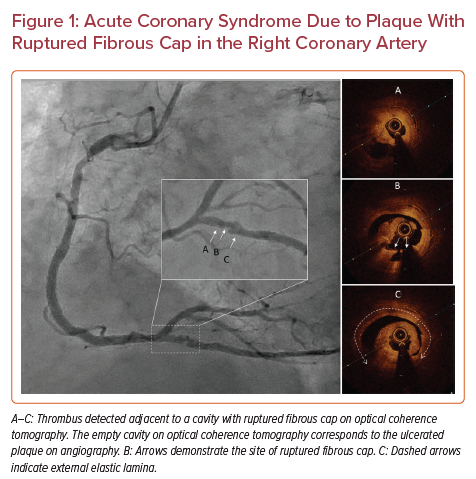
ACS due to plaques with RFC (Figure 1) portends a worse prognosis than plaques with IFC.20,21 RFCs occur in TCFAs.22–24 An OCT-based study indicated that patients with ACS due to ruptured TCFAs were more likely to have lesions in the proximal segments of the three major coronary arteries.25 Another OCT-based study in patients without ACS identified that TCFAs in these patients did not have a particular pattern of distribution.26 TCFAs are the most common pathological precursor for RFCs, but are associated with a relatively low risk of ST-elevation MI (STEMI) and sudden cardiac death.27,28 In addition, a study using OCT found that patients with lipid-rich plaques were at increased risk of cardiac events, especially if the lipid-rich plaques were longer, wider, and caused higher degrees of stenosis.29
Plaques can destabilize without presenting clinically if the thrombus is not completely flow limiting, in which case the plaques can undergo normal healing after thrombus resolution.30,31 OCT can identify healed RFCs by visualizing layers with different optical density.32,33 Patients with stable angina have a significantly higher number of healed RFCs than patients with recurrent ACS (51.4% versus 75%; p=0.001), which can signify abnormal healing in patients with recurrent ACS compared with patients with stable ischemic heart disease.34
RFCs tend to be located in the proximal and middle segments of TCFAs (80.7% versus 19.3% in the distal segment).35 RFCs in the distal segment of TCFAs are associated with a larger thrombus volume (4.50 versus 2.02 mm3; p=0.027) and a higher incidence of no reflow (31.7% versus 12.8%; p=0.003).35 Previous studies using OCT and IVUS found that patients with diabetes were more prone to plaques with RFC than those without diabetes; however, no consistent association between the frequency of plaque rupture and diabetes was observed in larger studies.36–38 OCT-based assessment has identified a relationship between ACS and the circadian rhythm. ACS is most likely to occur at 9 am, with the risk of RFCs increasing between 6 am and 11.59 am (OR 2.13; p=0.002), as well as between 12 pm and 5.59 pm (OR 2.10; p=0.005) compared with the interval between 12 am and 5.59 am.39 These results only apply on weekdays (p=0.01), supporting the catecholamine-surge hypothesis as an inciting event in the pathogenesis of RFCs. The same study found no circadian rhythm for ACS secondary to IFCs and calcified nodules.39
Plaques with Intact Fibrous Caps on OCT
IFCs visualized with OCT can be placed into two categories: ‘definite’ or ‘probable’ culprit plaques with IFC.40 A luminal thrombus in contact with a visualized plaque is considered a definite plaque with IFC, whereas an irregular lumen without a thrombus is considered a plaque with probable IFC. A culprit plaque is also considered probable IFC if it contains a thrombus without superficial calcification adjacent to the thrombus.41 IFCs are less likely to be associated with TCFAs than RFCs and are more likely to form in the left anterior descending artery and equally likely to form in the circumflex artery compared with plaques with RFCs, whereas plaque rupture is more common than IFCs in the right coronary artery.24 Both IFCs and RFCs tend to occur in the proximal/middle segments of the coronary arteries.24 RFCs portend an increased risk of multivessel disease and are more likely to be associated with an initial Thrombolysis in Myocardial Infarction (TIMI) flow ≤1, whereas IFCs are associated with higher-grade TIMI flows.24,42
On OCT, IFCs tend to have less diameter stenosis, lower lipid content, thicker caps, smaller lipid arcs, and less calcification than RFCs.36,43,44 IFCs form near branching areas in younger female patients without cardiovascular risk factors.36,43 Disruption of the laminar flow is thought to play a role because branching areas are strongly correlated with IFC formation.45 OCT-based studies suggest that neutrophils and CD8+ lymphocytes activated in response to transition from the laminar to turbulent flow may lead to endothelial damage and the formation of IFCs.43,45
IFCs that manifest as ACS are associated with more lipid-rich plaques, macrophage accumulation, and calcification in patients presenting with TIMI flow ≤1.20 Compared with non-lipid-rich plaques, lipid-rich plaques, defined as a maximum lipid arc >90° and lipid length >1 mm, were associated with higher rates of cardiac death and target vessel revascularization in ACS due to plaques with IFC at a medium follow-up of 21 months (p=0.002).20 Macrophage infiltration causes IFCs to become unstable, resulting in more negative outcomes, including sudden cardiac death and target vessel revascularization at 2.5 years.46
In a single-center non-randomized uncontrolled study of patients with ACS secondary to OCT-defined erosion (n=55), patients with residual diameter stenosis <70% on coronary angiography were treated with antithrombotic therapy without stenting.47 Manual thrombectomy was performed prior to OCT acquisition in 46 (83.6%) patients, 35 patients received glycoprotein IIb/IIIa inhibitors plus aspirin, and all patients received ticagrelor.47 Of the 55 patients, 53 completed follow-up at 12 months, with no major adverse cardiac events observed in 49 (92.5%).47 Three patients (5.7%) needed revascularization with stenting due to stable angina, and one patient (1.9%) had gastrointestinal bleeding.48 Most patients (52 of 53) completed further follow-up at a median of 4.8 years, with no incidence of death, MI, stroke, bypass surgery, or heart failure observed.49 Eleven patients (21.1%) underwent elective target lesion revascularization during the follow-up period.49 On OCT, there was a larger reduction in the thrombus volume from baseline to 1 month in 41 patients who did not undergo revascularization compared with the 11 patients who underwent elective revascularization (95% versus 45%; p=0.001).49 The results of this study warrant a randomized controlled study to assess whether a no-stenting approach is optimal in treating patients with IFC-ACS.49
Calcified Nodules on OCT
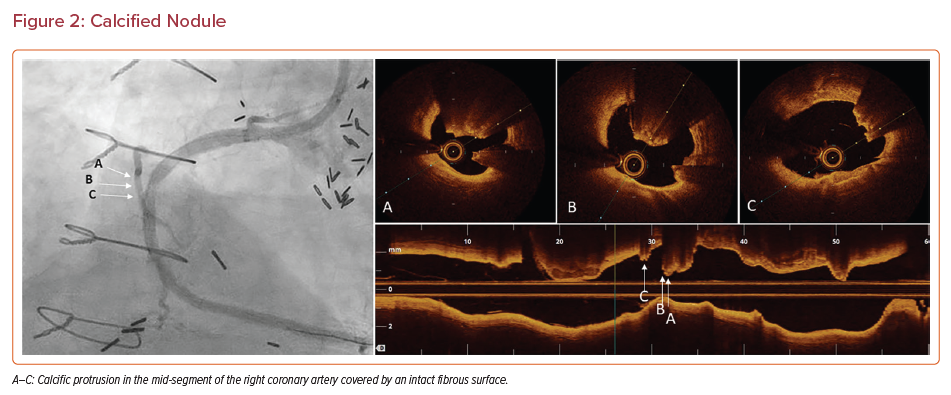
Calcified nodules can be classified into two groups: eruptive calcified nodules and calcified protrusions.50 Eruptive calcified nodules are defined as calcified nodules with a compromised covering fibrous layer that can cause obstruction of the lumen. Calcified protrusion is a calcific mass that, unlike eruptive nodules, is covered by a smooth leading edge that does not break through the fibrous surface of the plaque (Figure 2).50
Superficial calcified sheets are calcific plates without eruption into the lumen that are organized in a sheet-like pattern. A working hypothesis is that superficial calcified sheets may progressively evolve to calcified nodules and eruptive calcified nodules because of fractures that are generated due to the ongoing impact of cyclic mechanical forces.
Eruptive calcified nodules with a disrupted fibrous cap are associated with a sevenfold increase in the risk of death and MI compared with calcified protrusions with an intact fibrous cap.51 Eruptive calcified nodules are mostly observed in the middle segment of the right coronary artery, likely due to particular local flow dynamics and excessive hinge movement of the artery. Thrombi on eruptive nodules tend to be red, whereas thrombi associated with superficial sheets are commonly white, composed of fibrin and platelets.50,52 It is unclear whether calcified sheets are merely associated with thrombi or play a causative role in their formation.
The physical properties of calcified nodules make them challenging to treat. Compared with IFCs and RFCs, calcified nodules are associated with smaller stent areas because the eccentric rigidity of nodules impedes symmetric stent expansion.53 Eruptive nodules are associated with an increase in procedural complications, such as stent edge dissection and stent malapposition, and calcific sheets are associated with the smallest average post-stent area because of the often inadequate lesion preparation in the context of ACS.54 Small stent diameter and area continue to limit the effectiveness of PCI, and atherectomy may be needed to optimize outcomes in calcified lesions. As atherectomy is contraindicated in thrombotic lesions in ACS, intravascular lithotripsy could be considered because it uses a different mechanism to fracture the calcium; nevertheless, studies on the safety and efficacy of intravascular lithotripsy in patients presenting with ACS are warranted.55
Utility of OCT to Guide Percutaneous Coronary Intervention in ACS
In addition to the diagnostic utility of OCT in differentiating RFCs from IFCs and eruptive calcified nodules, OCT can be useful in determining vessel size, plaque structure and burden, which could be useful information in performing PCI in patients with ACS.
Although the following OCT studies have been limited due to retrospective design, lack of long-term clinical outcome, and modest differences, they help illustrate culprit plaque morphology in ACS. A randomized study of 141 patients presenting with STEMI found that manual thrombectomy did not improve the effective flow or minimum stent area, whereas a retrospective OCT-based study reported a relationship between post-thrombectomy residual thrombus and the extent of microvascular dysfunction.23,56 A 2:1 propensity-matched, prospective cohort study found a larger minimum lumen diameter in 214 patients with STEMI treated with pre- and post-stenting OCT guidance compared with 428 patients treated by angiographic guidance only (mean [±SD] diameter 2.99 ± 0.48 versus 2.79 ± 0.47 mm; p<0.0001).57 OCT guidance led to higher post-PCI fractional flow reserve compared with angiographic guidance in 240 patients presenting with non-STEMI (0.94 ± 0.04 versus 0.92 ± 0.05; p<0.005).58 A retrospective analysis of 11,731 patients in South Korea’s AMI Registry Database included 2,659 patients who were treated with intravascular imaging guidance (22.7%), with IVUS used in 2,333 (19.9%) patients and OCT in 277 (2.4%). Compared with the angiography guidance cohort, patient-oriented composite endpoints of all-cause death, MI, and revascularization were lower in the intravascular imaging cohort (7.7% versus 5.9%, respectively; HR 0.74; 95% CI [0.60–0.92]; p=0.006).59
Utility of Intravascular Ultrasound to Guide Percutaneous Coronary Intervention in ACS
IVUS has a lower resolution than OCT in identifying microstructures (necrotic core, fibrous cap, erosions) and thrombi; thus, its utility is overall less in ACS compared with OCT, and so this review has primarily focused on the utility of OCT in ACS. Nonetheless, IVUS can be useful in ACS when an alternative etiology apart from plaque disruption leads to the clinical manifestation. For example, a retrospective study examining 38 patients presenting with MI showed that culprit lesions in the infarct-related artery are distinct from non-culprit lesions in the same artery and plaques in a non-infarct-related artery.60 In that study, 16 (42.1%) patients presented with STEMI.
Thrombi were more common in culprit plaques than in infarct-related artery non-culprit plaques or non-infarct-related artery plaques.60 The frequency of ruptured/dissected plaques was similar in all three locations. Culprit lesions were longer, had larger external elastic membrane and plaque plus media cross-sectional area but smaller cross-sectional area, and more often exhibited positive remodeling than did infarct-related artery non-culprit plaques and non-infarct-related artery plaques.60 There was a trend for culprit plaques to be less eccentric than non-culprit plaques (mean [±SD] 0.4 ± 0.3 mm2, 0.3 ± 0.2 mm2, and 0.3 ± 0.3 mm2, respectively; p=0.08). The two control groups (i.e. the non-culprit plaques in the infarct-related artery and the non-infarct-related artery plaques) were similar.60
Utility of Coronary Physiology to Guide Percutaneous Coronary Intervention in ACS
Coronary physiology is an important tool in guiding the management of patients with ACS.61 In STEMI there is usually no need for assessment of the culprit vessel.61 The non-culprit vessels can be assessed with physiology, and flow-limiting lesions treated with intravascular imaging-guided PCI.61 In patients with non-STEMI, physiology can be combined with intracoronary imaging, especially for complete functional revascularization.61 Finally, intracoronary physiology is paramount in the assessment of microvascular dysfunction during ACS.61
Non-Atherosclerotic Causes of ACS
ACS is most commonly secondary to atherosclerotic lesions; nevertheless, rarer, non-atherosclerotic etiologies can also cause ACS, including spontaneous coronary artery dissection (SCAD), coronary artery spasm, and coronary embolism. In this section we review these conditions and discuss the role of OCT in their evaluation.
SCAD is the non-iatrogenic, non-traumatic, often asymptomatic separation of the coronary vessel wall that mostly underlies ACS in middle-aged women (i.e. between 44 and 55 years of age).62,63 The pathophysiology involves an inciting event (i.e. rupture of the vasa vasorum) that disrupts the vessel wall, leading to the formation of hematoma.56 Separation can occur at the proximal edge of the hematoma, but can also occur at random because blood travels through the dissection plane with minimal resistance. Dissection can extend in either direction, with the mean length of dissection exceeding 45 mm on angiography.64 Although rupture of the vasa vasorum is considered the inciting event, visualization with OCT has shown no difference in the density of the vasa vasorum in the healthy and dissected portions of the vessel.65
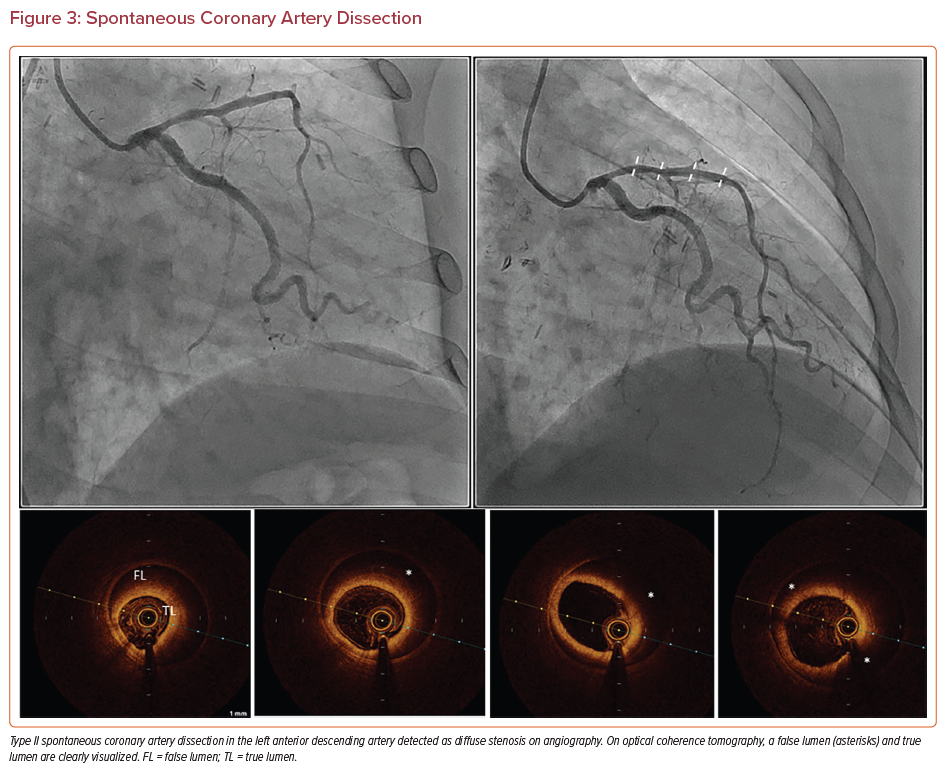
All cases of SCAD involve the formation of a false lumen that may compress the true lumen and present as angina, cardiogenic shock, or sudden cardiac death.63,66 SCAD can be classified as three subtypes. Type I SCAD appears as contrast staining in the arterial wall and is associated with a radiolucent lumen, making angiography sufficient for the diagnosis.63 Type II SCAD is the most common subtype, and involves diffuse stenosis of variable length and severity (Figure 3). Affected areas appear as a sudden change from a normal to narrow diameter. Type III SCAD is <20 mm in length.63
Type II and III SCAD require OCT and IVUS imaging for diagnosis. After vasodilators are given to exclude vasospasm, visualization with OCT provides a clear image of the intimal tears, intraluminal thrombi, false lumen, and hematoma; however, if the hematoma extends deeper into the vessel wall, OCT will not capture the entire structure due to limited penetration depth.67 Nevertheless, OCT should be used sparingly to diagnose SCAD because contrast injection can hydraulically propagate the dissection.63 With IVUS there is less risk of propagation, and the entire hematoma can be visualized. Nevertheless, angiography and IVUS are associated with increased risks of intimal tearing and extension of the dissection and should be performed carefully and with meticulous technique.
SCAD is usually treated with conservative medical therapy and lifestyle modifications; nevertheless, revascularization with PCI or bypass may be needed in unstable patients. In these cases, IVUS is used to guide entry into the true lumen and deliver the stent. Longer stents (5–10 mm longer than the lesion) are used to avoid propagation of the dissection when deployed.63 A distal stent may be deployed first, followed by a proximal stent to cover the segments with SCAD to prevent propagation.68
Coronary Artery Spasm
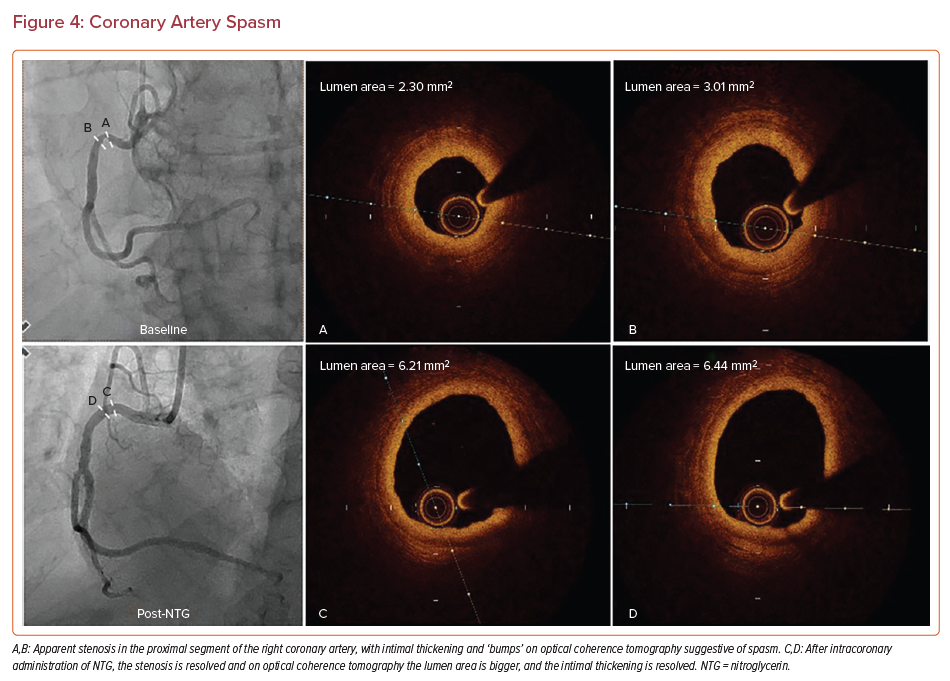
Coronary artery spasm can occur in healthy or diseased segments of coronary arteries.69 Smokers and adults aged between 40 and 70 years are at an increased risk of developing spasm.70 Coronary vasospasm is included in the differential diagnosis if a patient presents with MI and non-obstructive coronary arteries.71,72 Studies using intravascular imaging in patients with vasospastic angina and unstable angina have indicated that patients with spasm tend to have less plaque burden, more negative remodeling, fewer TCFAs, and cores with less nectoric features.73 On OCT imaging in patients presenting with vasospastic angina, luminal irregularity (which appears as bumping of the intima) was observed (Figure 4).74 Of note, in one-quarter of the spastic sites there were erosions with thrombus, indicating that erosion could be a secondary pathology in patients with vasospasm.75 Thus, antiplatelet therapy should be considered in patients presenting with vasospastic angina to prevent the risk of thrombus and embolization.
Coronary Artery Embolism
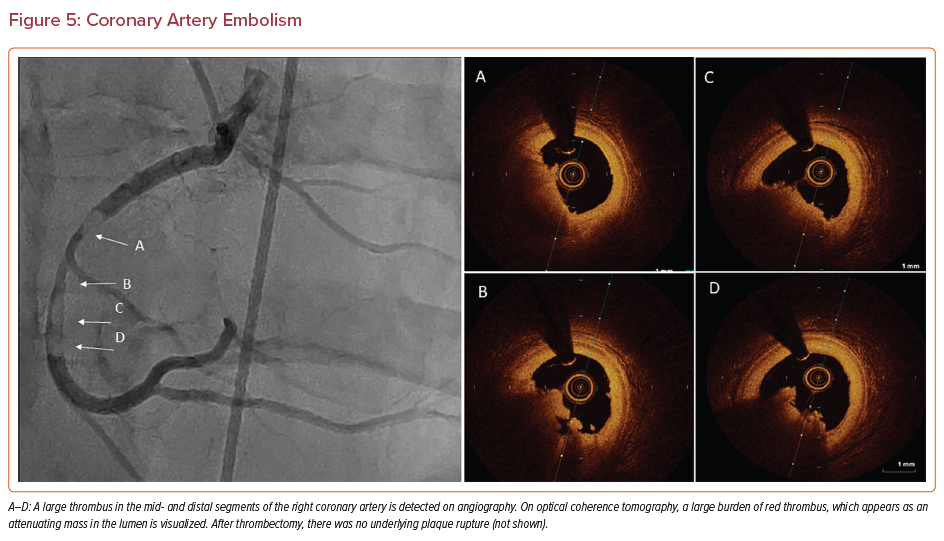
Coronary artery embolism is underdiagnosed and leads to preventable deaths. It is estimated that coronary emboli are responsible for 3% of all cases of ACS.76,77 The embolized material can be thrombus (Figure 5), tumor, air, vegetation, or foreign material, all of which can manifest as an embolus that obstructs blood flow to the myocardium.78
Coronary emboli can be direct, paradoxical, or iatrogenic.78 An embolus that forms within the left side of the heart is considered a direct embolus, whereas a paradoxical embolus forms in the deep veins of the leg.78 Paradoxical emboli enter the right heart, cross through a defect in the interventricular septum, and can obstruct the coronary vasculature. Iatrogenic emboli may occur during PCI if air enters into the arterial structures and can precipitate obstruction of blood flow.71 Patients with thrombophilia, AF, valvular disease, patent foramen ovale, infective endocarditis, and non-bacterial thrombotic endocarditis are predisposed to coronary emboli.78 Appropriate diagnosis and management of these underlying conditions can reduce the risk of future coronary emboli in these patients.
Coronary embolism appears as a heavy thrombus burden with an abrupt occlusion on angiography.76,79 If the occlusion happens before the major branches, several coronary territories can become ischemic. The unaffected vessels look normal and will not have collateral circulation because emboli present acutely. After thrombectomy, OCT or IVUS is performed to confirm that there is no underlying atherosclerosis to precipitate MI.76,79 This is an essential step in diagnosis and management.
OCT in the Diagnosis of MI with Non-obstructive Coronary Arteries
Patients who present with MI and are found to have unobstructed arteries on angiography are said to have MI with non-obstructive coronary arteries (MINOCA). A multimodal approach consisting of OCT and cardiac MRI is needed to diagnose the etiology of MINOCA. In a study of 40 patients with MINOCA, 10 (25%) patients had normal coronary arteries, 18 (45%) had lumen irregularities, and 12 (30%) had mild stenosis <50%.80 OCT was performed in the same 40 patients and revealed that 32 patients (80%) had substrates that predisposed them to ACS.80
In a larger study, of 170 women diagnosed with MINOCA, 145 had adequate OCT image quality for analysis and, of these, 116 underwent cardiac MRI.74 A definite or possible culprit lesion was identified by OCT in 46.2% (67/145) of participants, most commonly plaque rupture, intraplaque cavity, or layered plaque. Cardiac MRI was abnormal in 74.1% (86/116) of participants. An ischemic pattern (infarction or myocardial edema in a coronary territory) was present in 53.4% (62/116) of participants undergoing cardiac MRI. A non-ischemic pattern (myocarditis, takotsubo syndrome, or non-ischemic cardiomyopathy) was present in 20.7% (24/116) of participants. A cause of MINOCA was identified in 84.5% (98/116) of women with multimodality imaging, higher than that with OCT (p<0.001) or CMR (p=0.001) alone.74 An ischemic cause was identified in 63.8% of women with MINOCA (74/116), a non-ischemic cause was identified in 20.7% (24/116) of women, and no mechanism was identified in 15.5% (18/116) of women. Thus, multimodality imaging with OCT and cardiac MRI identified potential mechanisms in 84.5% of women with a diagnosis of MINOCA.
Limitations
The studies discussed in this review have several limitations. They are largely retrospective, limited to the centers with a high level of expertise rather than routine clinical use, and may be different from contemporary technologies. In addition, OCT is not optimal in poor TIMI flow and patients with advanced renal dysfunction due to the requirement for contrast use, and, in SCAD, may propagate coronary dissection.
In the 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography guideline for coronary artery revascularization, the use of intravascular imaging is recommended (Class 2a) or IVUS in procedural guidance, particularly left main and complex coronary stenting, with OCT as an alternative option to IVUS, except in ostial left main disease.81 In patients with stent failure, IVUS or OCT is recommended (Class 2a) to determine the mechanism of stent failure.81 There has been no specific mention of the use of intracoronary imaging in that guideline regarding ACS.
Conclusion
Intracoronary imaging provides additional data with regard to the underlying causes of ACS (Supplementary Material Figure 1). Approximately half the culprit lesions in ACS are secondary to plaques with RFCs, whereas erosions account for approximately one-third and eruptive calcified nodules for 5%. Less common, non-atherothrombotic causes of ACS that may be overlooked with angiography alone include SCAD, spasm, and coronary emboli. OCT is key in a multimodal approach to the diagnosis and management of MINOCA. Existing data suggest that intracoronary imaging may be useful in optimizing PCI in ACS, but randomized trials are warranted to substantiate a role for intravascular imaging guidance in improving the long-term outcomes after PCI in ACS.