Plato said, “The beginning is the most important part.” This adage applies to interventional cardiology, particularly to complex percutaneous coronary intervention (PCI). The success of a complex coronary program depends on meticulous vascular access. In this article, we describe various aspects related to this topic including access selection, technique, closure, and monitoring, as well as the prevention and management of various complications.
Access Site Selection
Radial
Contemporary studies examining the benefits of radial access in acute coronary syndrome (ACS), including RIVAL, RIFLE, and MATRIX, show a theme of improved mortality and lower major adverse events (albeit defined slightly differently in each), with numerically lower rates of bleeding and vascular complications in some trials.1–3 A recent meta-analysis confirmed that radial access reduced major bleeding in patients, including those with stable ischemic heart disease (79% reduction; 95% CI [48–92%]) and ACS (40% reduction; 95% CI [34–53%]).4 Additionally, radial access improved both major vascular complications in all-comers and overall mortality for patients with ACS.
Given these data and recent guideline updates, radial artery access has become the default option for diagnostic coronary angiography and intervention. Consideration of right versus left radial access depends on patient-specific factors (namely, left or right internal mammary grafts, patient stature, patient age, and known arterial malformations such as arteria lusoria), and the presence of catheters shaped to cannulate both coronaries from the right-radial approach.
Traditional radial access has been supplanted in some instances by distal radial access for improvement of patient comfort and ergonomics, especially in left radial access. Distal radial access is also thought to lower rates of radial artery occlusion (RAO), as suggested in a meta-analysis from 2022, which showed a 64% reduction in RAO with distal radial access, but with a 138% increase in access failure.5
The use of ultrasound in vascular access has also become the preferred standard of care based on the RAUST trial, in which ultrasound guidance reduced the number of attempts needed from >3 to <2 (mean 1.65–1.2 versus 3.05–3.4, p<0.0001), increased first pass success rate from 43.9% to 64.8% (p<0.0001), decreased time to access from 108 to 88 seconds (p<0.0006), and reduction in the need for femoral crossover from 5.7% to 1.4% (p=0.007).6
In recent years, several additional options have become available to facilitate complex PCI in patients with severe innominate/subclavian artery tortuosity or arteria lusoria, including longer sheaths (75–85 cm) for additional support/torqueability. However, their internal diameters are currently limited to 6 Fr.
Ulnar
Ulnar artery access provides an additional option for access; in some series, one in five patients is ulnar dominant, with a larger ulnar artery than radial.7 As such, when the radial artery appears small on ultrasound, the ulnar artery is imaged. If the radial is small but patent and the ulnar is larger, the ulnar is cannulated. Given the lack of a bone behind the ulnar artery, the single wall technique is usually used. Additionally, when applying the closure band, after seeing a ‘flash’ of blood at the arteriotomy as air is withdrawn, 5 ml of air is added instead of the usual 3 ml. The use of the radial and ulnar arteries simultaneously has been described but is generally not recommended because of the risk of hand ischemia.8
Femoral
Femoral access is occasionally required when using larger catheters (i.e. 8 Fr guiding catheters for intravascular ultrasound [IVUS]-guided proximal cap puncture or because of previously failed chronic total occlusion PCI), absent radial arteries (i.e. after use for coronary artery bypass grafting), failed radial access, and/or to place mechanical circulatory support (MCS). As such, maintaining proficiency in femoral access remains paramount for those performing coronary interventions.
The combination of fluoroscopic and ultrasound guidance is crucial in minimizing access site bleeding and vascular complications. On fluoroscopy, the inferior margin of the femoral head is marked with a hemostat/marker. After this, using ultrasound, the artery is ‘scanned’ to identify the bifurcation of the superficial femoral artery (SFA)/deep femoral artery (DFA) (marking the inferior border of the common femoral artery [CFA]) and the inferior epigastric (rarely seen, but if visible, marking the superior border of the CFA). Under ultrasound guidance, we usually attempt to enter the CFA approximately 1 cm above the bifurcation using a ‘mini-stick’ kit at a 45° angle (25–30° when MCS is to be used) in a single-wall fashion. After wiring, but before placing the sheath, we perform fluoroscopy to ensure that the needle tip is in the lower half of the femoral head (ideally the lower third) to reduce the chances of a ‘high stick.’ The mini stick sheath is then advanced, and angiography is performed to confirm that the sheath is in the CFA below the inferior epigastric but above the bifurcation prior to upsizing. Femoral closure is described separately but is made much easier by successfully cannulating the CFA at a site away from significant plaque or calcification, for which ultrasound guidance is invaluable.
Brachial
Historically, brachial artery access was performed using surgical cut down and direct arterial puncture, but radial, ulnar, and femoral access have largely replaced brachial access. Percutaneous brachial artery access should be reserved for impossible radial/ulnar and femoral artery access, but this remains an important option when alternative access sites are not available.
The brachial artery is smaller than the femoral artery. The average diameter was reported to be 3.69 ± 0.57 mm in women and 4.95 ± 0.64 mm in men in the Framingham Offspring Study cohort.9 The distal brachial artery is ideal for access, as the artery in this location separates from the nerve bundle and is located above the medial epicondyle of the humerus, allowing better manual compression for successful hemostasis.
Meticulous closure and post-procedure monitoring are very important because of the smaller space in the arm, in which minor internal bleeding or hematoma can result in compartment syndrome. The median nerve is in proximity, making nerve injury a risk. The risk of major complications has been reported to be 6.5–9.0%, with common complications including hematoma, compartment syndrome, transient numbness due to nerve injury, pseudoaneurysm, and distal limb ischemia due to thrombus formation, embolization, or stenosis at the site of the arteriotomy.10,11 As with other forms of vascular access, ultrasound, smaller sheath sizes, prompt anticoagulation, and judicious vasodilator use can help lower complication rates.
Axillary
Transaxillary access is growing in popularity for use in structural heart interventions and MCS placement in patients without adequate iliofemoral vessels. Historically, surgical graft implantation was required for large bore axillary access, but percutaneous access and closure devices are associated with less morbidity than surgical techniques requiring general anesthesia. The choice of axillary access is based on vessel size, patient hand dominance, aortic arch type with attention given to retroflexion of the innominate artery, and avoiding puncture through pacemaker or ICD pockets.
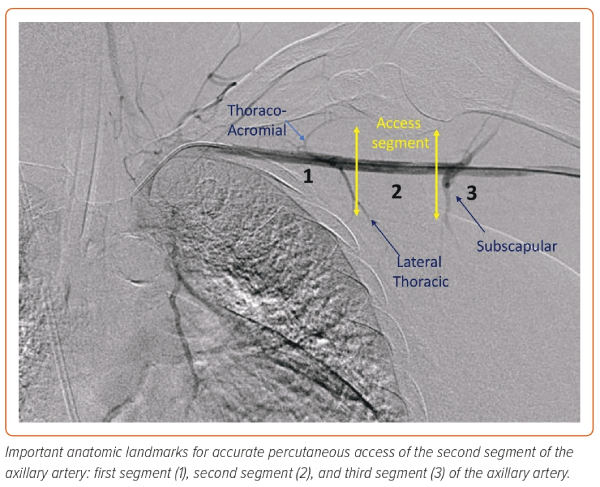
The axillary artery measures 5–8 mm in diameter. It is infrequently affected with atherosclerotic disease compared with the femoral artery.12 The suitability of the axillary artery for use can be assessed by CT or with angiographic and ultrasound imaging at the time of the procedure. Preprocedural planning and proper patient and room position are crucial for successful access. The table should have an arm extension, and the patient’s arm should be extended and abducted. A second access point and placement of a 0.018" wire across the axillary artery, or alternatively roadmap angiography, is useful to delineate the course of the artery. The axillary artery is divided into three segments in relation to the pectoralis minor muscle. The target zone is the second segment lateral to the thoraco-acromial branch and medial to the subscapular branch (Figure 1). A shallow angle of entry is recommended to allow large sheath placement as it courses between the clavicle, as a steep angle of entry may result in kinking of the sheath, a common reason for access failure or closure difficulty. Closure methods are similar to those for femoral artery closure.
Ultrasound Guidance
As with radial access, ultrasound guidance has become recommended for femoral artery cannulation based on the FAUST trial, which demonstrated that the use of ultrasound guidance improved first pass success rate versus no seconds (83% versus 46%; p<0.000001) and reduced the number of attempts (3.0–1.3; p<0.000001), median time to access (136 seconds versus 148 seconds; p=0.003), and vascular complication rates (1.4% versus 3.4%; p=0.041).13 Rates of low cannulation were reduced, but there was no difference in high cannulation; as such, we use both fluoroscopy and ultrasound.
Familiarity with ultrasound characteristics and techniques are key to successful access. Imaging should be performed using a vascular probe with evaluation in both longitudinal and cross-sectional views. The longitudinal view allows needle visualization from skin to artery but can be difficult and requires an experienced provider, as the imaging arrays must be perfectly aligned with the needle course. The longitudinal view allows clear visualization of the bifurcation and the extent of arterial calcification. The cross-sectional view is most often used in obtaining access and allows differentiation of artery from vein and visualization of the adjacent structures. Veins will be compressible and thin walled, while arteries will be non-compressible, thicker-walled, potentially calcified/atherosclerotic, and pulsatile. The use of color Doppler (CD) can help distinguish artery versus vein based on direction and pulsatility of flow. Furthermore, CD can confirm patency of arteries before access is attempted, to prevent inadvertently accessing occluded vessels. A thorough evaluation of the degree, extent, and location of calcification and intra-luminal plaque are crucial, because entry into a calcified segment is associated with increased complications and vascular device closure failure. Avoiding entry into an atherosclerotic segment will help prevent dislodgement and subsequent athero-embolization or subintimal tracking because of entry within the plaque.
Micropuncture Technique
The clinical application of smaller gauge needles for arterial access is appealing because of the reduced arteriotomy size. Standard femoral arterial access needles are 18 gauge (G) compared with the 21G micropuncture needles. The cross-sectional area of the arteriotomy is reduced by 56% using micropuncture. The smaller arteriotomy created by micropuncture allows faster time to hemostasis compared with larger needles.14 The potential improved safety profile in cases of malpositioned vascular puncture sites has been the main reason for adoption of micropuncture in cardiac cath labs.
Despite the preference for micropuncture at many hospitals, there remains limited research to support its clinical utility. Two large observational studies failed to demonstrate a reduction in overall vascular complications with the use of micropuncture. Mignatti et al. reported a non-statistically significant trend in reducing access-site hematomas and pseudoaneurysms.15 A larger retrospective study of 17,844 patients was conducted with 2,344 patients using micropuncture (18G) and 15,500 patients having a standard (21G) needle used for vascular access.16 Access complications were fewer with the use of micropuncture compared with the standard needle (2.5% versus 3.6%; p=0.005), predominantly driven by lower rates of access-site hematomas with micropuncture (1.4% versus 1.9%; p=0.03). There were no differences reported between the two techniques regarding rates of arteriovenous fistulas, retroperitoneal bleeding, pseudoaneurysms, or limb ischemia.
Several attempts have been made at conducting randomized clinical trials (RCTs) evaluating micropuncture against standard vascular access. Unfortunately, the two most contemporary RCTs were terminated early because of low clinical event rates and loss of funding.
Radial Sheathless Techniques
When the radial artery is smaller than 2.8 mm, or when an 8 Fr guide is required, sheathless guide catheter techniques are recommended for safer radial use. Dedicated 7.5 Fr sheathless guide catheters (Asahi Intecc; Supplementary Figure 1) have been proven to be safe and effective.17 Additionally, 8.5 Fr sheathless guide catheters (Asahi Intecc) are only available outside the US. Supplementary Figure 2 displays the feasibility and safety of transradial PCI using a 6.5 Fr sheathless guide catheter in patients with small radial arteries.
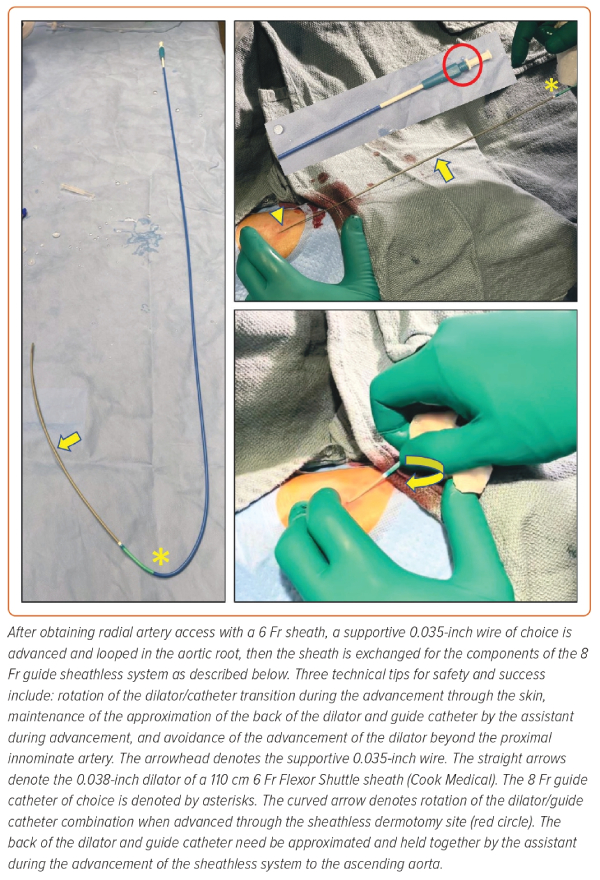
If dedicated 7.5 Fr sheathless guide catheters are not available, one may telescope either a 5 Fr 110 cm pigtail catheter, a 5 Fr 125 cm multi-purpose catheter, or the 0.035" dilator of a 5 Fr 110 cm Flexor sheath (Cook Medical) in any 90 or 100 cm 7 Fr guide catheter over a 0.035" wire, in a sheathless fashion. If operators wish to maintain the benefits of radial access in complex PCI requiring an 8 Fr guide, they could then telescope either a 6 Fr 110 cm pigtail catheter, a 6 Fr 125 cm multi-purpose catheter, a 6 Fr 125 cm dedicated carotid diagnostic catheter, or an 0.035" dilator from a 6 Fr 110 cm Flexor sheath in any 8 Fr guide catheter shape over an 0.035" wire in a sheathless fashion (Figure 2).
Limb Perfusion
In any instance where a large bore sheath is left in place (i.e. for MCS), ensuring limb perfusion is essential, as limb loss is independently associated with higher morbidity and mortality in this patient population (Figure 3A–B).18 Although it is possible to perfuse the affected leg without antegrade femoral access using internal contralateral femoral-profunda bypass, this technique is complex and requires expert peripheral vascular skills, time, and radiation.19 For these reasons, it is usually only performed when the ipsilateral SFA is severely diseased or occluded. In most cases, leg perfusion involves obtaining antegrade femoral access.
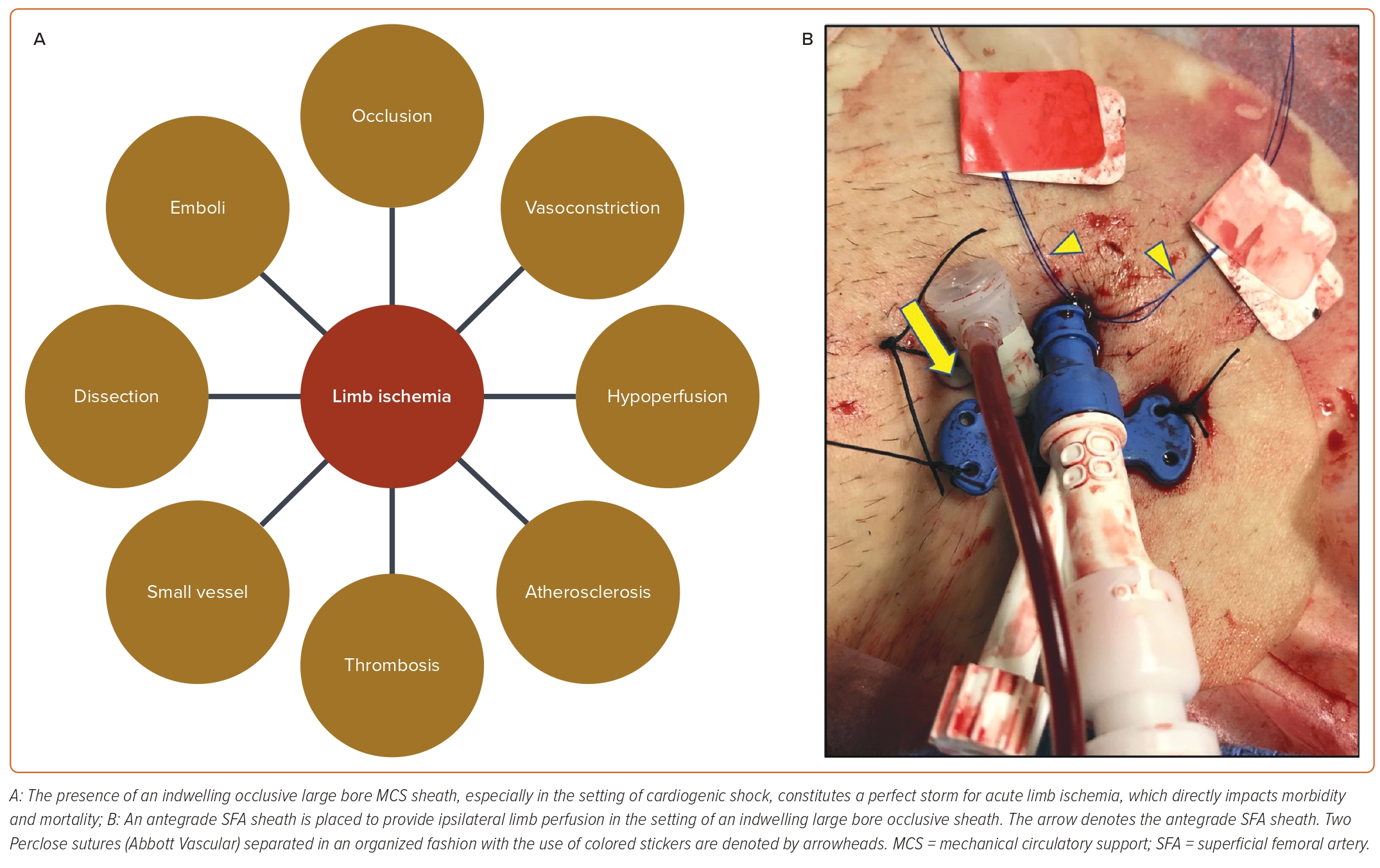
In non-urgent cases, it is preferable to obtain antegrade access of the ipsilateral limb prior to the placement of an occlusive large bore sheath. This relates to the difficulty in fitting an ultrasound probe in the space under a large bore sheath to allow adequate visualization of the CFA or proximal SFA for antegrade access. Placement of an ipsilateral antegrade femoral sheath does not usually interfere with the ability to obtain retrograde CFA access or with upsizing the retrograde femoral sheath.
Although some operators prefer to access the lower CFA in antegrade fashion, then wire the SFA, this potentially lengthy technique is not favored because of the tendency of the access wire to go into the DFA and less room available for re-wiring the SFA under fluoroscopic/ultrasound guidance. Most operators currently access the proximal SFA under ultrasound guidance, as has been proven safe for endovascular interventions.20
Once antegrade femoral access is achieved, a perfusion circuit is established by connecting the side port of the sheath to the side port of a donor sheath with a male-to-male connector. Donor sheath options are multiple, resulting in many external bypass options: contralateral retrograde CFA resulting in the external contralateral femoro-femoral bypass technique(Supplementary Figure 3A); ipsilateral retrograde CFA large bore sheath resulting in the external ipsilateral femoro-femoral bypass technique (Supplementary Figure 3B); and ipsilateral retrograde radial artery access resulting in the external radial-femoral bypass technique or ‘Lend a Hand’ technique (Supplementary Figure 3C).19,21
Large Bore Closure
Dry Closure
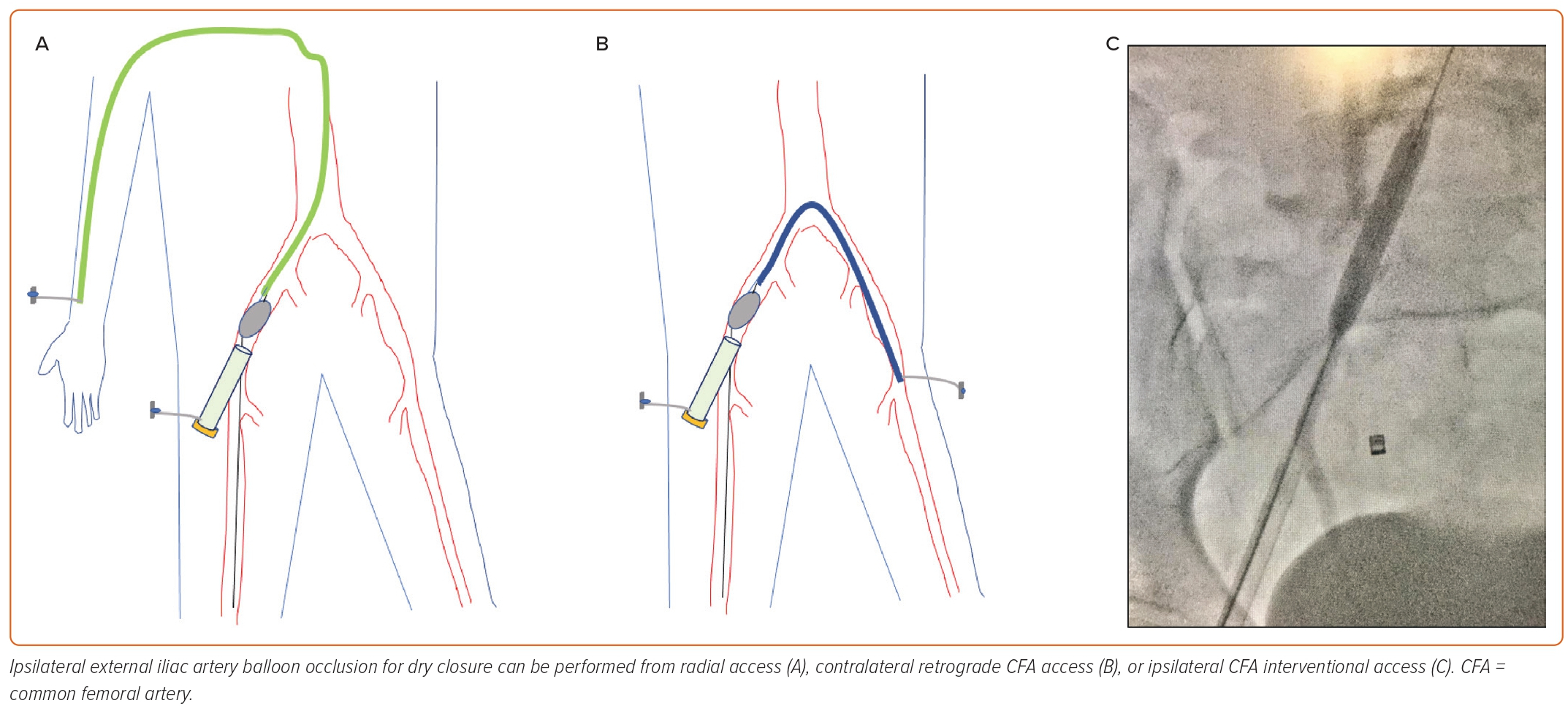
Some operators closing large bore arteriotimies advocate for dry closure of large bore sheaths through balloon occlusion of the ipsilateral external iliac artery via a secondary access point, minimizing bleeding and allowing time to optimize closure, improving clinical outcomes (Figure 4A–C). This is traditionally achieved by obtaining contralateral femoral or radial access and sheath placement to advance/inflate a 1:1 balloon sized to the iliac vessel at low pressure. This works well but adds the morbidity of an additional vascular access along with requiring additional time, iodinated contrast, radiation, and peripheral vascular skill sets. Additionally, with the single access technique becoming preferred in most practices, the risk–benefit ratio of obtaining an additional vascular access for the purpose of dry closure has become less favorable.22
A technique has been described to allow dry field closure without the additional burden of another access.23 The single access dry closure technique (Figure 4) involves the delivery of an occlusion balloon into the ipsilateral external iliac artery through the retrograde CFA large bore sheath, inflating at low pressure, then pulling the large sheath out over the shaft of the occlusion balloon while the non-locking Perclose (Abbott Vascular) suture is pulled to approximate the edges of the arteriotomy. Angiography can be performed via the balloon in order to confirm the absence of vascular complications; once confirmed, the balloon is then deflated and pulled out in a relatively dry field, and the Perclose sutures are tied.23
Pre-closure
Traditional pre-closure for sheaths larger than 8 Fr is performed with two Percloses. After obtaining CFA access as described above, and before introducing large sheaths (i.e. a 14 Fr sheath for percutaneous ventricular assist device insertion), a single Perclose is deployed (usually at the ‘10 o’clock’ position) and then a second Perclose deployed offset by 90–120° (usually at the ‘2 o’clock’ position); these sutures are then secured in that position via hemostat to the drape. Care must be taken not to pull on the locking suture (non-rail, or white suture) during this process so as not to ‘air tie’ the suture. The blue suture (rail) can be pulled on, though, to improve hemostasis. At this point, the large sheath may be advanced.
At the completion of the procedure, over a 0.035" wire, the large sheath is removed. The first Perclose (at 10 o’clock) is deployed by applying the snare knot pusher to this knot. The second Perclose (2 o’clock) is then tightened in a similar fashion. Once hemostasis is ensured and the 0.035" wire is removed, the knots are locked in the same order, and the sutures are then cut in the same order. If two Percloses do not provide adequate hemostasis, as long as the 0.035" wire remains in, an additional device (usually an 8 Fr Angio-Seal [Terumo Interventional Systems]) can be deployed atop the two Percloses in order to aid hemostasis, with manual compression hemostasis remaining an option throughout.
Several publications have also explored the use of a single Perclose for pre-closure; one of the largest, published in 2020 by operators at the Cleveland Clinic, found that single pre-closure was adequate in nearly 25% of patients, with the balance of the patients needing additional closure with an Angio-Seal.24
Post-closure
Post-closure of large bore indwelling sheaths has been made more straightforward in recent years through the addition of a 0.035" wire side arm/rewire port on Impella sheaths (Abiomed), obviating the need to cut the Impella and wire the purge port in order to maintain access to the femoral vasculature. Our usual approach to post-closure is to perform femoral angiography through the sidearm/rewire port, especially if placement was not performed at our institution, to ensure CFA access. If low or high, vascular surgical consultation is obtained. If the device is in the CFA, we use a 0.035" wire (usually an Amplatz Super Stiff [Boston Scientific] or Lunderquist [Cook Medical]) to remove the Impella. Once out, an 8 Fr sheath is placed into the arteriotomy site over the first wire. Through this, another wire – usually the access wire – is advanced, and with these two wires in place, another 8 Fr sheath is placed in the femoral artery. At this point, closure is performed with a Perclose, one sheath at a time, while keeping a dilator in the remaining sheath at the time of deployment of the first Perclose in order to avoid suturing into the sheath wall. Maintenance of a 0.035" wire throughout this process is paramount, as there may be residual bleeding requiring the deployment of an Angio-Seal device to ensure patent hemostasis. Additional post-closure techniques describing the use of two Angio-Seal devices have been described but are not routine at most centers.25 Several purpose-built large bore closure devices also exist, of which the most used is the MANTA device (Teleflex Incorporated), which does not require pre-closure and does facilitate post-closure.
Monitoring Protocols
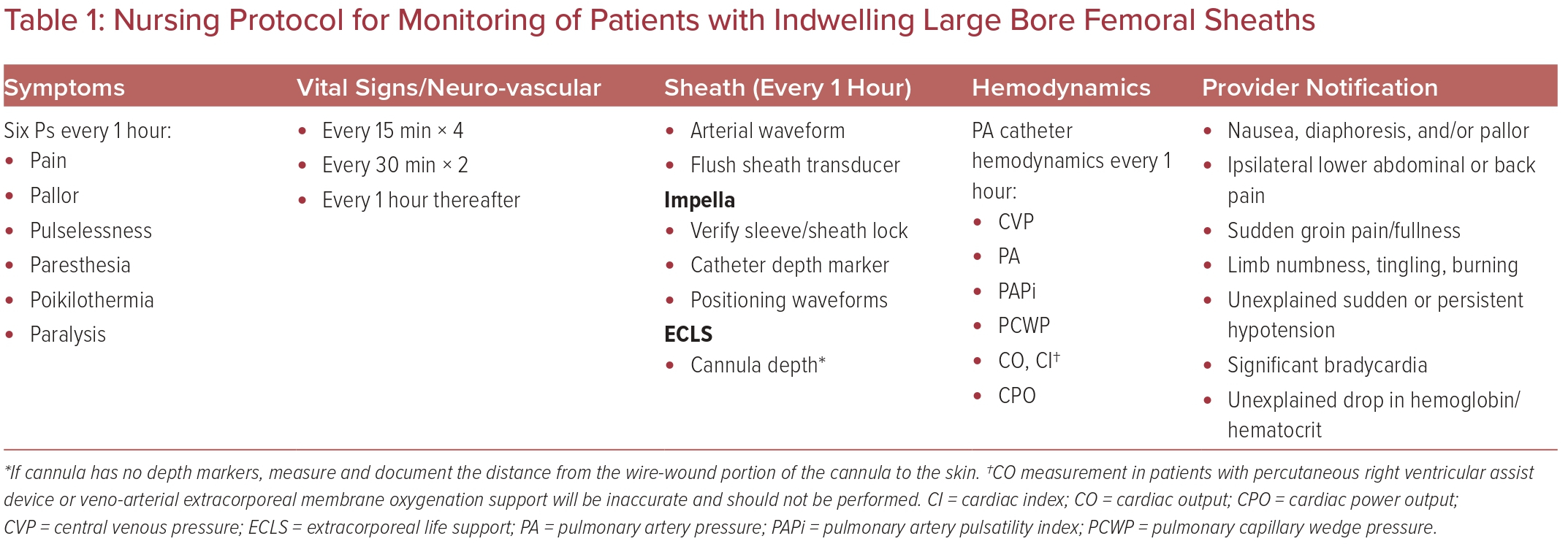
Arterial bleeding complications post-procedure can quickly become a life-threatening emergency. Close observation by nursing staff in a critical care setting can aid in rapid recognition of post-procedure bleeding and patient deterioration. Our institutional monitoring protocols are detailed in Table 1. Sheaths and cannulas used for MCS (Supplementary Table 1) are much larger than typical sheaths used for coronary interventions, increasing the risk of vessel occlusion and limb ischemia. The laminar flow pattern of these devices further complicates physical assessment, as pulse pressures may be diminished or absent depending on the amount of blood flow generated by the device and the underlying contractility of the native heart.
Patients undergoing MCS support in the setting of low or absent pulse pressures may require vascular Doppler assessment if pulses are not palpable. If signs of limb ischemia are observed in the setting of audible Doppler signals, the perfusion pressure of the limb may be measured by inflating a sphygmomanometer proximal to the probe. A pressure of <50 mmHg is diagnostic for limb ischemia and should be addressed immediately.26
The Extracorporeal Life Support Organization (ELSO) recommends the routine use of distal limb perfusion catheters for any patient with a 17 Fr peripheral arterial cannula or larger. ELSO also recommends use of continuous near-infrared spectroscopy (NIRS) to monitor distal limb perfusion. Target tissue oxygen saturation values of ≥50%, with a <20% difference between the cannulated and non-cannulated limbs, denotes adequate perfusion.27 NIRS monitoring may provide an earlier warning of impaired tissue perfusion with higher sensitivity than physical or Doppler assessment, especially in the setting of high-dose vasopressor support. Several studies using NIRS have demonstrated preserved distal tissue perfusion in the setting of absent Doppler signals and poor physical exam, thus avoiding unnecessary attempts at distal perfusion catheter placement and potential associated bleeding complications.28,29 Alternatively, the use of ankle-brachial index (ABI) in extracorporeal membrane oxygenation (ECMO) patients undergoing continuous NIRS monitoring has been described; ABI decreases of 0.10–0.15 were associated with acute limb ischemia without concomitant changes in NIRS values, but this was not statistically significant.30 Thus, until further studies can elucidate the optimal modality of limb monitoring, an assortment of techniques are recommended in the presence of large bore indwelling arterial sheaths to ensure early detection of impaired tissue perfusion. In the event of disagreement between monitoring techniques, a multidisciplinary approach is recommended to aid interpretation of conflicting findings.
If antegrade limb perfusion is employed (Figure 3), it is imperative that the nurse frequently inspects the external bypass circuit for signs of impaired flow. Flow may be inspected by placing a Doppler probe over the external tubing or by visual confirmation of flow at the sidearm via flashlight. Clotting of the external bypass circuit will result in visual changes within the tubing. The circuit may darken as clot interrupts flow. Separation of blood and plasma may also be observed in the setting of prolonged stagnant flow. Pressure tubing should be employed to connect the donor and recipient sheaths to prevent inadvertent kinking of the circuit.
Anticoagulation
Intra-Aortic Balloon Pump
The decision to use anticoagulation should be considered in the context of other indications or contraindications and risk of bleeding. However, with augmentation frequency of 1:2 or lower, patients should receive therapeutic anticoagulation.
Impella
Use of unfractionated heparin (UFH) containing purge solution prevents thrombus formation within the Impella device. Concomitant use of intravenous UFH is recommended with the Impella to prevent thrombus formation at the sheath site, in the Impella, or on the body of the Impella. The recommendation for the initial purge solution is a heparin concentration of 25 U/ml in 5% dextrose. In some patients, titratable supplemental intravenous UFH is needed to provide optimal anticoagulation considering the units of heparin in the purge solution and flow rates (total heparin minus heparin in purge solution). An anti-Xa target range of 0.2–0.4 IU/ml or an activated partial thromboplastin time (aPTT) corresponding to this anti-Xa range based on local laboratory values is recommended.31
In patients with suspected or confirmed heparin-induced thrombocytopenia or a heparin allergy, heparin-free purge solution is recommended with an alternative systemic anticoagulant such as bivalirudin or argatroban.32 Direct thrombin inhibitors (bivalirudin and argatroban) are not recommended for use in the purge solution.31
Careful monitoring of activated clotting times (ACTs) is important to reduce the risk of bleeding. If clinically significant bleeding or coagulopathy develops, a stepwise approach would follow in which the first step is the discontinuation of systemic anticoagulation while maintaining heparin in the purge solution.
Veno-arterial Extracorporeal Membrane Oxygenation
Usage of veno-arterial ECMO (VA-ECMO) is associated with bleeding and thrombosis. However, because of the relatively higher risk of thrombosis, maintaining therapeutic anticoagulation is necessary.33 Currently, there is no universal protocol for anticoagulation in patients requiring VA-ECMO. Systemic anticoagulation with UFH is the mainstay, titrated according to ACT, aPTT, or anti-Xa test results.34 ELSO guidelines recommend an initial heparin infusion rate of 7.5–20.0 U/kg/h for anticoagulation during VA-ECMO.35
Management of Complications
Bleeding
Bleeding remains the Achilles heel of large bore access; however, the use of ultrasound guidance and radial access has significantly decreased this complication. Bleeding can be divided into access site and non-access site and compressible or noncompressible.
For access site bleeding, manual compression remains the usual treatment. Once hemostasis is achieved, switching to a compression device (i.e. a Femostop; Abbott Vascular) may be appropriate. Rarely, if hemostasis is not achieved through manual/mechanical compression, urgent peripheral angiography may be needed with potential coiling or covered stenting performed to stop bleeding.
One of the main reasons for close intensive care unit monitoring of this population is also the rapid detection of non-compressible access site bleeding (i.e. retroperitoneal; Table 1). It is imperative to resist the urge for stat imaging studies (i.e. a CT scan), since this would be a relatively more difficult environment for aggressive hemodynamic stabilization or running a code. If conservative measures are ineffective in controlling bleeding, peripheral angiography and intervention may be indicated as above. Rarely, open vascular surgical procedures may be needed.
Non-access site bleeding (i.e. gastrointestinal, ocular, spontaneous rectus sheath hematomas) is usually because of systemic anticoagulation; this is becoming less prevalent with the decrease in use of glycoprotein IIb/IIIa inhibitors. A high clinical suspicion must be maintained for unexplained hypotension or drops in hemoglobin, as gastrointestinal pathology may be unmasked with therapeutic anticoagulation. Intracranial/intraparenchymal hemorrhage remains a feared complication, for which urgent/emergent neurological/neurosurgical evaluation is warranted.
Dissection
Risk factors for access-related dissections include larger sheaths, smaller size vessel, iliac tortuosity, moderate or severe iliofemoral calcification, inadvertent manipulation and advancement of guide wires, sheaths, and catheters, high or low puncture site, and access at a bypass graft anastomosis.36 Routine femoral angiography immediately after access leads to early diagnosis, procedural planning, and rapid management of this complication. The management of dissections depends on the extent, direction (antegrade versus retrograde), and whether the dissection is flow-limiting or non-flow-limiting.
Non-flow-limiting Dissections
Antegrade blood flow usually heals non-flow-limiting retrograde iliac and femoral dissections. Anticoagulation is usually not indicated in non-flow-limiting dissections. Conservative management with follow-up clinical examination and noninvasive imaging such as Doppler ultrasound or CT angiography may be considered based on the clinical situation.
Flow-limiting Dissections
Endovascular techniques are the preferred option for the treatment of flow-limiting dissection. After obtaining contralateral femoral, radial, or brachial artery access, IVUS should be used to confirm the guide wire position in the true lumen. Self-expanding stents are commonly used for external iliac artery dissections, and balloon expandable stents are used for common iliac artery dissection.36–38 Because of the high risk of stent fracture, CFA dissection should be treated with prolonged balloon inflation at 1:1 diameter, and self-expanding stents in this location are reserved for failed balloon angioplasty only if surgical repair is too risky.39 Local catheter-directed thrombolysis followed by balloon angioplasty or stenting may be needed when thrombotic occlusion occurs with flow-limiting dissection. Open surgical repair may be needed in complex dissections, especially at CFA or bypass graft anastomotic sites.36–38 Stent thrombosis after treatment of dissections with stents has been reported; therefore, short term use of heparin with dual antiplatelet therapy may be considered in patients treated with stents.
Thrombosis
Thrombosis at access site vessels can occur because of flow-limiting dissection, during or after hemostasis with crossover balloon-inflation for dry closure, and because of the occlusive nature of relatively large bore sheaths, especially with vessel diameters <5.0 mm.40 Management approaches include maintaining therapeutic or higher ACTs, balloon angioplasty, thrombectomy (manual or mechanical) via contralateral or alternative access, stenting if concomitant flow-limiting dissections are present, and, in rare cases, catheter-directed thrombolysis,37 even though this is associated with a high risk of access site bleeding. If those measures fail, surgical revascularization may be needed.37 Arterial thrombosis can cause distal embolization, resulting in reduced perfusion or occlusion of the outflow vessels causing acute limb ischemia.38,40 Limb ischemia can also develop following removal of arterial cannulae because of dislodgement of thrombus that has developed around the cannula. After sheath removal, it is a good practice to perform angiography of the iliofemoral artery and distal runoff to at least the tibio-peroneal trunk to confirm distal vessel patency. If flow occlusion is anticipated based on vessel diameter as assessed by ultrasound or initial femoral angiography, antegrade access should be performed to allow for external bypass, and target ACT should be maintained for 200–220 seconds, as described above.
Pseudoaneurysm
Arterial pseudoaneurysms occur when a hematoma remains in connection with the arterial lumen, leading to a usually pulsatile area of blood at the site of arterial cannulation. Pseudoaneurysms may occur because of ineffective hemostasis or failure of vascular closure devices.41 Patients usually present with pain or swelling over their access site. On auscultation, a bruit may be heard, and a pulsatile mass may be appreciated. The diagnosis is usually confirmed with ultrasound showing a ‘yin-yang sign’ on Doppler, confirming blood flow into and out of the pseudoaneurysm. For smaller pseudoaneurysms, manual compression is the treatment of choice. Alternatively, injection of the pseudoaneurysm with thrombin has a success rate nearing 100% in some series.42 For large pseudoaneurysms, covered stenting and/or vascular surgical repair remain suitable options. By using ultrasound, micropuncture techniques, and appropriate closure techniques pseudoaneurysms can hopefully be avoided, preventing the need for treatment.
Radial Artery Occlusion
A complication limited only to radial/ulnar access, upper extremity distal arterial occlusion is rare in the modern era, seen in 2% of patients 3 months after radial artery access.43 In this study, radial artery occlusion (RAO) was associated only with higher rates of collateral flow, potentially leading to less recanalization in those with well-developed collaterals. The use of periprocedural anticoagulation – usually with unfractionated heparin – and patent hemostasis reduce the chance of RAO. Generally, RAO is well tolerated, as most patients are asymptomatic; however, those without collateral flow (i.e. patients with ipsilateral ulnar artery occlusion or an incomplete superficial palmar arch) may develop hand ischemia. Recanalization may be beneficial for these patients.44 Finally, the use of distal radial access has been associated with 62% lower rates of RAO (95% CI [43–75%] reduction) and presents an intriguing option for those patients in whom RAO is a concern.45
Conclusion
Vascular access is the first crucial step for any interventional procedure, and safe access is key to achieving optimal periprocedural outcomes. Therefore, careful planning of vascular access, considering a patient’s clinical status, vascular anatomy, and technical aspects of the intervention, is mandatory in securing procedural safety and successful results. Approaching every vascular access in a systematic and consistent way, even in emergent situations, allows operators to minimize the risk of complications in this increasingly sick patient population.