The use of bioprosthetic surgical aortic valve replacements (SAVR) has been steadily increasing in people aged 50–70 years over the past decade.1 This trend has been driven by the desire to avoid long-term anticoagulation and the development of novel percutaneous treatment options for valvular heart disease. Current-generation bioprosthetic valves remain prone to structural valve deterioration and have finite durability. This has significant implications for the younger, low-risk populations whose life-expectancy may exceed that of the initial surgical valve.2 While redo SAVR has traditionally been the gold standard for the treatment of failed surgical valves, valve-in-valve (ViV) transcatheter aortic valve replacement (TAVR) has arisen as a viable, less invasive option with the potential for improved short-term morbidity and mortality, but is only approved by the Food and Drug Administration for patients at high surgical risk for reoperation.3 The term ViV TAVR describes several clinical scenarios, including TAVR inside of a degenerated surgical valve (TAVR-in-SAVR), TAVR inside of a degenerated TAVR valve (TAVR-in-TAVR), and even TAVR inside of a TAVR valve, which was previously placed in degenerated SAVR valves (TAVR-in-TAVR-in-SAVR).4 We provide a review of clinical outcomes associated with ViV TAVR, procedural planning recommendations, and strategies to overcome technical challenges that can occur during ViV TAVR.
ViV TAVR Outcomes
The available retrospective registry data regarding ViV TAVR outcomes have been encouraging. Initial data from the 2014 Valve-in-Valve International Data (VIVID) registry, which pooled patients with both stenotic and regurgitant lesions, and those treated with balloon-expandable (BEV) and self-expanding valves (SEV), showed promising 30-day and 1-year survival rates of 92% and 83%, respectively.5
The PARTNER-2 multicenter registry, which included patients at high risk for mortality with re-operative surgery (average Society of Thoracic Surgeons score 9.1 ± 4.7%) treated with ViV TAVR using the SAPIEN XT BEV (Edwards Lifesciences), demonstrated sustained performance with no change in gradients, effective orifice area, or aortic regurgitation, and improvement in quality of life and functional status at 3 years.6 Of note, the PARTNER-2 registry excluded patients with SAVR valves <21 mm, limiting the applicability of this data to patients with small annuli.5,6 A recent propensity-matched registry analysis comparing early and late outcomes between SAVR and ViV TAVR showed lower 30-day mortality with ViV TAVR compared with SAVR, as well as improved survival at 5 years (76.8% versus 66.8%; HR 0.55; 95% CI [0.30–0.99]; p=0.04).7
While ViV TAVR is currently approved only for high surgical risk patients, a recent registry study examining lower-risk ViV patients treated with Sapien 3 BEVs showed comparable 30-day and 1-year outcomes to patients undergoing native TAVR. Based on a propensity-matched analysis, 30-day all-cause mortality in the low-risk (Society of Thoracic Surgeons <4%) ViV group was comparable to native TAVR (1.0% in the low-risk group versus 1.3% in native TAVR, p=0.44). Furthermore, 1-year all-cause mortality was actually lower in the ViV group compared with native TAVR (6.1% versus 8.5%, p=0.05).8 While further study is required, the data may open the door to an expansion of the indication for ViV TAVR to lower-risk patients.
Preprocedural Planning for ViV TAVR
Initial assessment of the indwelling bioprosthetic valve via cardiac multidetector CT may be the most important part of the procedure. The size, valve type (stented, sutureless, stentless), and leaflet orientation (externally or internally mounted leaflets) of the surgical valve need to be carefully assessed and factored into the choice of transcatheter valve. The ViV app (UBQO) is an essential source for ViV planning, providing information regarding the fluoroscopic appearance of various valves, true inner diameter measurements, and suggestions for transcatheter valve sizing.
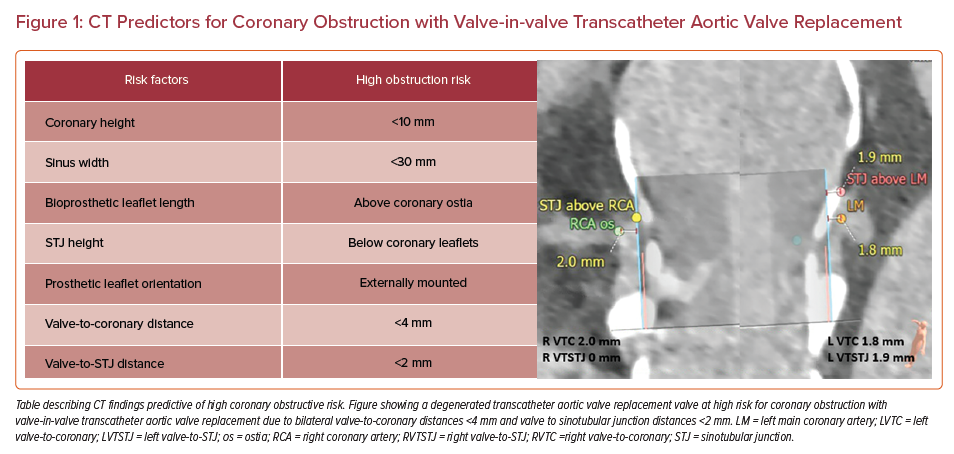
Careful measurements of the annular plane, coronary artery heights, sinotubular junction (STJ) height, and sinus of Valsalva sizes must be made, followed by implantation of a ‘virtual valve’ to estimate valve-to-coronary (VTC) and valve-to-STJ (VTSTJ) distances (Figure 1).9 The VTC, VTSTJ, leaflet lengths, and relationship of the leaflet tips of the existing surgical valve to the coronary ostia and STJ will help determine the risk for coronary obstruction.9,10 A VTC <3–4 mm and VTSTJ <2 mm is considered high risk for coronary obstruction in the setting of ViV TAVR and warrants further, more complex procedural planning.11,12
Choice of Transcatheter Heart Valve for ViV TAVR
Selecting the appropriate transcatheter heart valve (THV) for ViV TAVR requires close attention to the individual patient’s anatomy, as well as a plan for lifetime valve management, with careful attention to the risk of coronary obstruction, feasibility of future coronary re-access, and hemodynamic results.
Coronary access after TAVR is a concern that applies to both BEV and SEV designs, and occurs more commonly with ViV TAVR than de novo TAVR.13 The height and intra-annular position of a BEV may be advantageous over the design of SEVs in regard to coronary re-access and risk for coronary obstruction during ViV TAVR or future redo TAVR procedures. In contrast, a SEV may allow for retrieval or repositioning if there is evidence of impending coronary obstruction, with the trade-off of the risk of leaflets of the supra-annular SEV reaching the STJ, thus making coronary re-access challenging and potentially prohibiting a future redo TAVR.14
Consideration for the feasibility of a future TAVR-in-TAVR or TAVR-in-TAVR-in-SAVR should also be considered for the younger and lower-risk populations, who may potentially require three valves in their lifetime.
In a CT analysis study, the coronary artery ostia originated below the top of the neo-skirt in 90% of SEV first cases, compared with 67% of BEV first cases. Additionally, the risk for technically impossible coronary re-access was estimated at 27% for SEV, compared with 10% for BEV.15 This issue may be further compounded if the transcatheter heart valve is within an existing SAVR frame.
While the BEV’s low frame height may be favorable for coronary access, its intra-annular design within an existing SAVR may result in poorer hemodynamics and long-term durability. The VIVID registry found elevated postprocedural gradients, defined as mean gradients >20 mmHg, more common after BEV ViV than SEV ViV (40% versus 21.3%;, p<0.0001). Furthermore, BEV appeared to perform even worse in small surgical valves (inner diameter <21 mm) with higher rates of elevated postprocedural gradients when compared with SEV (58.8% versus 20%; p=0.005) at 1 year.5 While these findings have not necessarily translated into obvious mortality differences, the long-term clinical implications need to be further elucidated.16,17
Pitfalls of ViV TAVR
Coronary Obstruction Risk and Mitigation Strategies
Coronary artery obstruction is a rare (<1%), but life-threatening, complication of TAVR, with mortality rates as high as 41%.11,13 Coronary artery obstruction occurs more frequently among patients undergoing ViV procedures than first-time TAVR, with rates in registries ranging from 2.4% to 3.5%.11,18 Obstruction can occur when the leaflets of the existing SAVR are displaced toward the coronary ostia or STJ during TAVR valve deployment. Risk factors include female sex, low coronary ostia (<10 mm), effaced sinuses (<30 mm), narrow valve to coronary distances (<4 mm), and VTSTJ junction distances (<2 mm).11,13 Stentless bioprostheses and stented bioprostheses with externally mounted valve leaflets have been shown to be independent risk factors for coronary obstruction.19 Careful attention with preprocedural CT analysis measuring the coronary heights, STJ height and diameter, and modeling with a virtual valve will determine ViV risk and feasibility, and may help determine the more favorable transcatheter heart valve for a patient’s unique anatomy.9,10
Techniques have been developed to mitigate the risk for coronary obstruction in high-risk patients without options for redo surgical aortic valve replacements. These include coronary protection with a guidewire and/or stent and bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR (BASILICA).
Snorkel Stenting
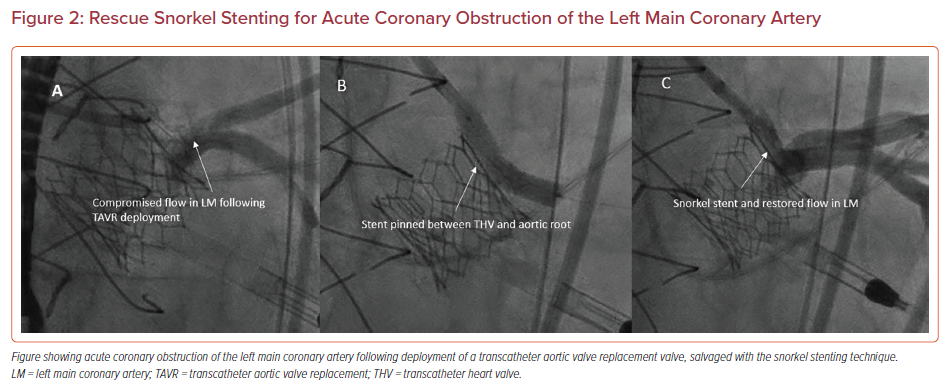
Snorkel or chimney stenting involves prepositioning a coronary wire and stent in a threatened coronary artery, and then deploying the stent in the ostium and ‘snorkeling’ it alongside the TAVR valve into the aorta if there is evidence of coronary compromise (Figure 2). These stents are exposed to continuous external compression from the TAVR valve, which places them at risk for delayed coronary obstruction with extremely challenging percutaneous bailout options.12 Data from the Chimney registry reported a 5% procedural death rate and 21.6% MI rate with this technique.20 Furthermore, techniques involving coronary protection by inserting a guidewire followed by removal once the TAVR valve is deployed and there is no immediate evidence of obstruction have also been associated with increased mortality and coronary obstruction.21
BASILICA
BASILICA, which uses radiofrequency energy to create longitudinal base-to-tip lacerations of bioprosthetic or native aortic valve leaflets, is a well-described alternative technique with more predictable and reliable outcomes.22
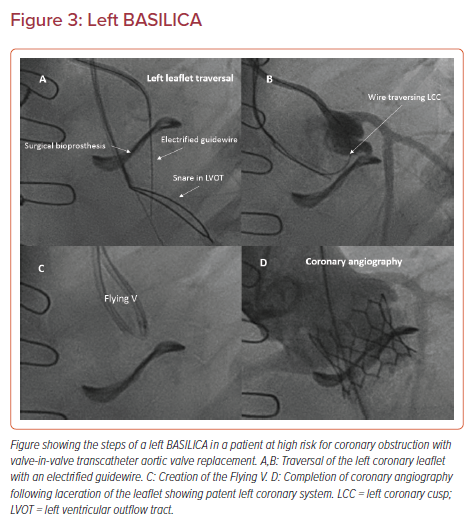
Briefly, BASILICA is typically performed under general anesthesia with transesophageal guidance, and standard transfemoral or transcaval access. Cerebral embolic protection is recommended in all cases with suitable anatomy. The target leaflet of the threatened coronary is traversed with a stiff, electrified guidewire (AstatoXS 20 or AstatoXS 40; Asahi) insulated with a hubless locking microcatheter (Piggyback Wire Converter; Teleflex) through a Pachyderm-shaped (PAL1/2/3 for left coronaries and PJR4 for right coronaries, Launcher; Medtronic) guiding catheter (Figure 3A).23 Of note, the dedicated Pachyderm catheters are no longer being manufactured. We recommend an AL2 or AL3 guide catheter for left BASILICA, and a JR4 guide catheter with a manually shaped tip for the right coronary artery. The guidewire is then snared in the left ventricular outflow tract, with careful attention to avoid trapping of the mitral chordal elements, and externalized to form a loop across the base of the leaflet (Figure 3B). This ‘Flying V’ lacerating surface is then positioned across the leaflet. The leaflet is lacerated in the standard fashion using a 5% dextrose solution during a 70 W continuous duty-cycle radiofrequency ablation (Figure 3C). Following successful laceration, a THV is deployed in the standard fashion and angiography is performed to confirm patency of the coronary artery (Figure 3D).
Data from the 2019 BASILICA trial demonstrated procedural success with this technique in 93% of patients, with 100% of patients free of coronary obstruction after TAVR at 30 days. There was a higher stroke rate (10%) than seen in original PARTNER-2 (6.4%) and SurTAVI (4.5%) trials, but in an extreme-risk group of non-operative patients.24 More recent data from the 214-patient multicenter International BASILICA registry has remained encouraging. Procedural success, defined as successful traversal and laceration without mortality, coronary obstruction, or emergency intervention, was achieved in 86.9% of patients, with a stroke rate of only 2.8% with judicious use of cerebral embolic protection.25
BASILICA does not address commissural malalignment or obstruction related to the skirt of the THV. BASILICA may also fail to prevent coronary obstruction in patients with challenging anatomy, such as very narrow VTCs (<2mm), diffusely calcified leaflets, and TAVR-in-TAVR procedures, due to inadequate leaflet splay despite otherwise successful leaflet laceration. An advanced BASILICA modification, balloon-assisted BASILICA, which involves inflating a coronary balloon in the target leaflet to widen the achieved splay, has been proposed to overcome these anatomical limitations.26
Additionally, there is a risk for leaflet prolapse into the coronary arteries potentially related to incomplete displacement or expansion of lacerated leaflets, or mechanical avulsion from excessive pull, rather than controlled laceration. A small case report suggests that leaflet prolapse may be more common in patients with stentless bioprostheses with mobile, degenerated leaflets.27
While BASILICA is an excellent tool to facilitate TAVR in patients otherwise considered poor candidates for ViV TAVR due to the risk for coronary obstruction, the technique must be adopted with caution and reserved for high-volume centers with experience using this technique. Currently the Food and Drug Administration does not support proctoring for BASILICA.
Patient–Prosthesis Mismatch
Patients undergoing ViV TAVR may be at risk for patient–prosthesis mismatch (PPM) due to constraints from the existing surgical valve, which can prevent full expansion of the new TAVR valve. PPM is defined as an effective orifice area of a prosthetic valve that is smaller than the orifice of the patients’ native aortic valve, with severe PPM defined as an indexed effective orifice area ≤0.65 cm2/m2.28
In the VIVID registry, the incidence of severe PPM following ViV TAVR was 31.8%, with reduced survival seen at 1 year (74.8%) in patients with a small surgical valve size (≤21 mm) compared with patients with an intermediate-sized valve (21–25 mm, 81.8%) or a large valve (≥25 mm, 93.3%). More recent data from a Sapien 3 ViV retrospective analysis did not show a difference in survival between those in a high postprocedure gradient group (≥ 20 mmHg) and those in a low postprocedure gradient group (<20 mmHg).
Proposed strategies to avoid severe PPM include the use of a supra-annular SEV, aiming for higher implant depths, and performing bioprosthetic valve fracture (BVF) in patients with small surgical valves and residual gradients >20 mmHg. The BVF technique involves positioning a non-compliant valvuloplasty balloon inside the frame of the existing surgical valve and then performing high-pressure inflation to fracture the sewing ring of the surgical valve, allowing further expansion of both the surgical valve and the implanted THV. Bench-testing performed by Saxon et al. and Allen et al. demonstrated the ability to reliably fracture Magna, Magna Ease, Mitroflow, Mosaic, and Biocor Epic bioprosthetic valves.29,30 A series of 20 patients undergoing BVF either before or after ViV TAVR showed a successful and significant reduction in mean gradient (20.5 ± 7.4 mmHg to 6.7 ± 3.7 mmHg; p<0.001), and increase in effective orifice area (1.0 ± 0.4 cm2 to 1.8 ± 0.6 cm2; p<0.001) with no procedural complications.31 This was again demonstrated in a larger 75-patient, multicenter study that demonstrated significantly lower gradients when BVF was performed, without complications.32 Recent data from 139 BVF cases performed at 11 centers demonstrated stable and excellent valve hemodynamics and survival at 30 days and 1 year.33
The ideal timing of when to perform BVF remains unclear. Fracture of the existing bioprosthetic valve first before implanting the THV may facilitate placement of a larger TAVR valve, although at the expense of potentially damaging the surgical leaflets and causing unstable aortic insufficiency. Attempting fracture following implantation of the THV may allow for a more controlled procedure due to less concern for hemodynamic instability, but may risk structural damage to the new THV leaflets and, therefore, risk early degeneration.29,31 The timing of BVF ultimately depends on operator comfort, aggressiveness of balloon sizing and dilatation, and requires further study regarding long-term clinical outcomes associated with this technique.
Valve Thrombosis and Anticoagulation Strategies
Clinical valve thrombosis after ViV TAVR is common, with rates as high as 7.6% in an analysis of 300 patients from the VIVID registry. This appears to be markedly reduced in patients taking oral anticoagulants (OAC) for other reasons (1.0% versus 11.3% in patients not taking OAC).34
Current guidelines recommend single antiplatelet therapy after TAVR with aspirin 75–100 mg daily for patients without co-existing indications for long-term anticoagulation.35 The POPular TAVI trial, which compared single antiplatelet therapy with dual-antiplatelet therapy after TAVR, did not show a reduction in thrombotic events with the more potent dual-antiplatelet therapy regimen, but did see more bleeding.36 Unfortunately, this study did not specifically evaluate the ViV TAVR population.
The recent ATLANTIS trial compared apixiban with standard of care post-TAVR, which was defined as either a vitamin K antagonist in patients with an indication for OAC or single antiplatelet therapy if there was no indication for OAC. Those treated with apixaban had a reduction in reduced leaflet motion and hypoattenuated leaflet thrombosis with the apixaban regimen, although this did not translate into an improvement in clinical outcomes at 30 days.37 Specific data for the ViV TAVR subgroup, comprising approximately 5% of each treatment arm, are not yet available.
Interestingly, some preliminary data have suggested that BASILICA may offer an additional benefit of reducing subclinical leaflet thrombosis by improving sinus washout and stasis.38 No hypoattenuated leaflet thrombosis was seen on TAVR leaflets adjacent to the lacerated aortic leaflets in the BASILICA trial.24
Until more data become available, the choice of antiplatelet or anticoagulation after ViV TAVR remains up to the discretion of the operator. Potential strategies to mitigate the risk of clinical and subclinical valve thrombosis included a cautious course of OAC in patients with low baseline bleeding risk, and/or leaflet modification strategies, such as BASILICA, to improve sinus washout. Further study is required before recommending a standardized therapy for all-comers following ViV TAVR.
Conclusion
ViV TAVR is associated with less morbidity and mortality than redo SAVR for patients with degenerated bioprosthetic surgical valves, but requires close attention to individual patient anatomy, as well as a plan for lifetime valve management with careful attention to risk for coronary obstruction, feasibility of future coronary re-access, and hemodynamic results. Procedural modifications, such as BASILICA and BVF, may be necessary to facilitate successful ViV TAVR procedures in high-risk patients.
Clinical Perspective
- Valve-in-valve transcatheter aortic valve replacement (ViV TAVR) is a viable, less invasive option for patients with degenerated aortic bioprostheses, with the potential for improved short-term morbidity and mortality when compared with redo surgical aortic valve replacement.
- ViV TAVR requires close attention to individual patient anatomy, as well as a plan for lifetime valve management with careful attention to the risk of acute coronary obstruction, feasibility of future coronary re-access, and hemodynamic results.
- The risk for coronary obstruction can be mitigated with careful preprocedural CT planning and the use of techniques, such as snorkel stenting or BASILICA.
- Bioprosthetic valve fracture may help address patient–prosthesis mismatch following ViV TAVR.
- Optimal anticoagulation strategies following ViV TAVR have not yet been elucidated.