Transcatheter left atrial appendage (LAA) closure has emerged as a non-pharmacological alternative to long-term anticoagulation for stroke prevention in appropriately selected patients with AF.
Over the past decade, multiple devices have been developed for transcatheter LAA closure, with continuous refinement in design and peri-procedural techniques. Watchman FIx and Amplatzer Amulet are the only two devices that have been approved by the Food and Drug Administration (FDA), whereas multiple other devices are under clinical trials in the US; however, some are CE mark approved, and under use in Europe and the rest of the world.
Watchman Flx Device
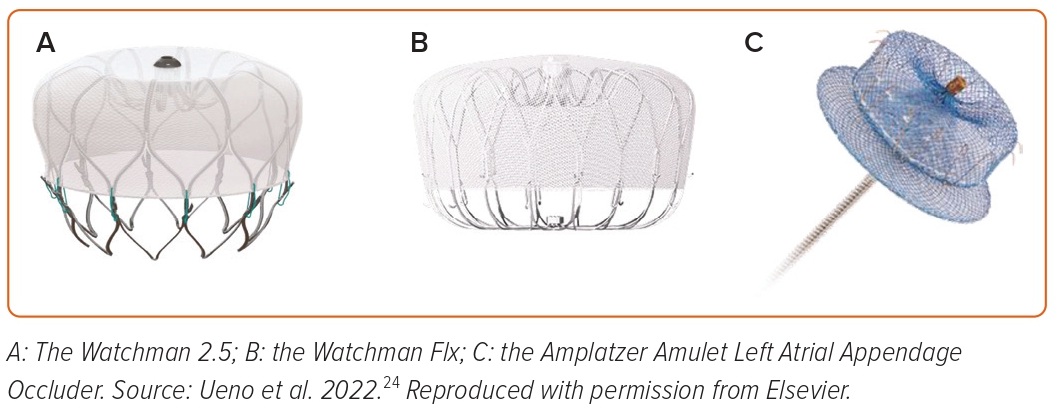
The Watchman 2.5 is now replaced by the Watchman Flx (Boston Scientific Corporation) device, the latest among the Watchman family devices (Figures 1A and 1B). It is a parachute-shaped, self-expanding nitinol device with 18 strut frames and 24 (12 in each row) J-shaped anchors. The polyethylene terephthalate membrane fabric extends distally on the device to achieve a better seal and reduce peri-device leaks (PDLs). The device comes in five different sizes (20, 24, 27, 31, and 35 mm) for LAA ostia measuring from 14 to 32 mm. The device can be fully recaptured, repositioned, and redeployed for optimal placement. The device is implanted using a 15 Fr Watchman FXD Curve sheath, which has better torque ability compared with previous sheaths.
The Watchman device is the most studied of all of the LAA closure devices. The most robust data for the safety and clinical effectiveness of the Watchman device in comparison with oral anticoagulation are available from the two FDA registration trials – PROTECT-AF and PREVAIL.1,2 A patient level, pooled meta-analysis of the PROTECT-AF and the PREVAIL trials at 5 years found that the Watchman 2.5 device showed similar efficacy for the prevention of ischemic stroke and systemic embolism compared with warfarin, with reduction in major bleeding and all-cause mortality.3 With increasing experience over the past years, as well as improvement in device design, the procedural steps have been optimized, leading to better safety outcomes. In the SURPASS analysis from the NCDR-LAAO registry, including 16,048 patients receiving a Watchman Flx device, the procedural success rate was 98%, 82% patients had no PDL, and the incidence of major procedural adverse events was 0.37%.4
Amplatzer Amulet Device
The Amplatzer Amulet occluder device (Abbott) is the newer-generation device following the earlier Amplatzer Cardiac Plug device. It is a self-expanding nitinol device that consists of a lobe and a disc connected by a central waist. Polyester patches are sewn into both the lobe and disc to facilitate occlusion. The sizing of the device is based on the landing zone, the intended location where the lobe of the device is placed in the LAA, usually measured 10–12 mm from the orifice. The device comes preloaded in eight different sizes ranging from 16 to 34 mm that can fit into LAA landing zone width from 11 to 31 mm. The Amulet device is implanted through a 12–14 Fr double-curved TorqVue sheath (Figure 1C).
In the Amulet IDE trial, which randomized 1,878 patients to either the Amulet occluder or Watchman 2.5 device, the Amulet occluder was non-inferior for safety and effectiveness of stroke prevention compared with the Watchman device.5 The LAA occlusion rates were higher with Amulet (98.9% versus 96.8%); however, the procedure-related complications were also higher compared with the Watchman device (4.5% versus 2.5%), which decreased with the operator experience.5 The SWISS APERO trial randomized 221 patients to either Amulet or Watchman (77.3% patients received Watchman Flx) across eight European centers.6 Amulet was not associated with a lower rate of the composite of crossover to a non-randomized device or residual LAA patency compared with Watchman at 45-day cardiac CT. However, Amulet was associated with lower PDL rates on transesophageal echocardiography (TEE), higher procedural complications, and similar clinical outcomes at 45 days compared with Watchman. Further studies comparing the Amulet device with Watchman Flx are awaited.
Ultraseal Device
The second-generation Ultraseal LAA occluder (Cardia) is a self-expandable nitinol device composed of a distal bulb, a proximal sail, and an articulating joint (Figure 2). The device is available in 10 sizes, ranging from 16 to 34 mm, and the length of the device ranges from 10 to 18 mm. The device has been modified from its earlier version in terms of having a lower radial force, making the bulb more flexible, reducing the length of the device by decreasing the length of the articulating joint, and modification of the sail polyester covering. A bulb-to-landing zone oversizing of 10–20% is recommended.
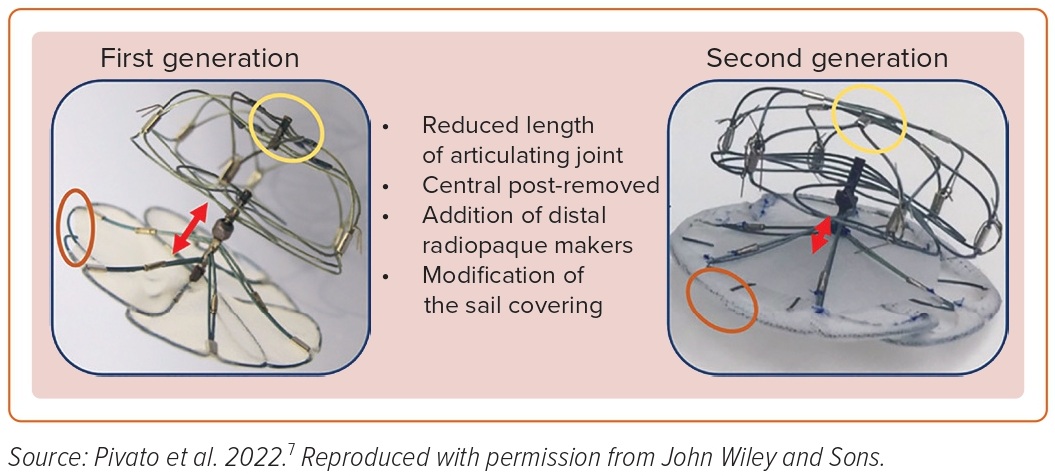
In a multicenter international registry including seven European centers and 52 patients undergoing second-generation Ultraseal device implantation, technical success was achieved in 96.1% patients.7 The primary safety outcome of in-hospital major adverse events, defined as a composite of all-cause death, stroke or transient ischemic attack, systemic embolism, major bleeding, MI, major vascular complication, or device embolization, was found in 5.8% of patients. In the study, 34 patients underwent follow-up TEE at a median duration of 61 days; 2.9% patient had a peri-device leak >5 mm and no patients had a device-related thrombus (DRT).7
Lambre Device
The Lambre device (Lifetech Scientific) is a self-expanding, nitinol-based device with a hook-embedded umbrella and a cover connected with a short central waist (Figure 3). The proximal cover is larger in diameter than the umbrella, sewn with polyethylene terephthalate fabric, and intends to cover the LAA orifice on deployment. The distal umbrella comprises eight claws with individual stabilizing hooks, as well as a polyethylene terephthalate membrane. The device comes in several sizes ranging from umbrella diameter 16–36 mm.
In a prospective multicenter study of 153 patients from 12 centers in China, the LAA was successfully occluded in 99.4% patients.8 The major peri-procedural complications were observed in 3.3% patients. At 12 months, the DRT rate was 1.3% and residual leak >3 mm was 0.8%.8
CLAAS Device
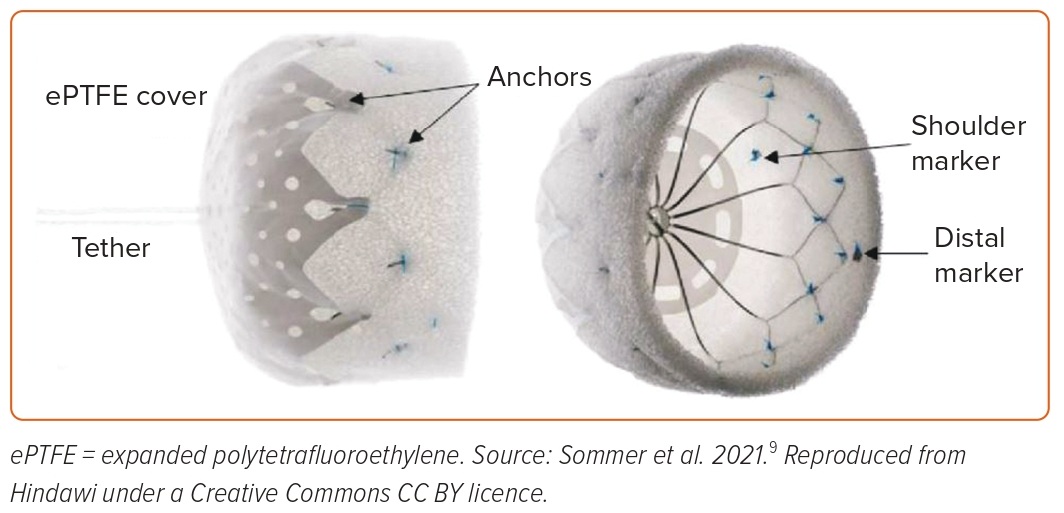
The CLAAS (Conformal Left Atrial Appendage Seal; Conformal Medical) device is a foam-based, self-expanding occluder consisting of a cylindrical nitinol endoskeleton covered with a conformable, porous, polyurethane-carbonate matrix foam (Figure 4). The endoskeleton has two rows of anchors for stability, whereas the porous foam cup has a polytetrafluoroethylene fabric cover to provide a thromboresistant outer surface. The device is available in two sizes: 27 mm for a mean LAA diameter ranging from 13 to 25 mm; and a 35-mm device, which can be used for a mean LAA diameter ranging from 20 to 32 mm. In a preclinical study of device implantation in seven healthy male canines in sinus rhythm, at 60 days, histologic examination showed complete neointima covering with minimal inflammation.9
In a report of 15 patients using intracardiac echocardiography-guided deployment of this device, the success rate was 100%, and there were no procedure/device-related complications. At 1 year, there was no significant peri-device leak (>5 mm), and DRT was detected in one patient at 6 months.10 The CONFORM Pivotal trial, a large-scale randomized controlled trial comparing the CLAAS device with the Watchman or Amulet devices, is ongoing (NCT05147792).
Several other devices, such as WaveCrest (Biosense Webster), Omega LAA occluder (Vascular Innovations), SeaLA LAA occluder (Hangzhou Valued Medtech), and LACbes (PushMed), are under clinical evaluation.
Limitations
Peri-device leak and DRT are the two major limitations of the current generation of intracardiac LAA closure devices. PDLs result in incomplete occlusion of the LAA, leading to risks for thrombus formation and systemic embolism. The most common reason for PDLs includes morphological discrepancies between occlusion devices and the LAA, suboptimal implantation of the device, expansile nature of the LAA, and anatomic remodeling of the LAA after device implantation.
The majority of the data pertaining to the PDL comes from the Watchman family of devices, since they have been the most commonly used and studied devices. In the initial clinical trials, a PDL ≤5 postimplantation, as well at 45-day follow-up, was accepted as sufficient LAA closure and the basis for discontinuation of chronic oral anticoagulation. However, newer data have shown that any PDL is associated with an increased risk for thromboembolic events. In a study of 1,054 patients from the PROTECT-AF, PREVAIL, and CAP2 (continues access to PREVAIL) trials using earlier-generation Watchman devices, the presence of PDL ≤5 at 1 year, but not at 45 days, was associated with a twofold increased 5-year risk of ischemic stroke or systemic embolism, largely driven by an increase in non-disabling stroke.11 In the NCDR-LAAO Registry analysis of 51,333 patients undergoing LAAO with the Watchman 2.5 device between 2016 and 2019, on post-implant TEE at 45 ± 14 days, 73.4% had no leak, 25.8% had small leaks (>0–5 mm), and 0.7% had large leaks (>5 mm).12 Interestingly, compared with patients with no leak, those with small leaks had higher odds of stroke or transient ischemic attack or systemic embolization (adjusted HR 1.15), major bleeding, and any major adverse events. There was a trend, but no significant differences, in adverse events between patients with large leaks and patients with small or no leaks; however, the results may be underpowered due to the small number of patients with large leaks.12 It is important to note that most of the studies on PDL used TEE as the imaging modality. In clinical practice, the use of cardiac CT for pre- and postprocedural assessment is gaining popularity; however, there is substantial variability in leak detection between the two modalities.13 Cardiac CT appears to be more sensitive than TEE in detecting, as well as characterizing, the morphology of PDLs.13,14
DRT is uncommon; however, it is associated with increased risk for ischemic strokes. In a meta-analysis of 66 studies, the overall incidence of DRT was ~4%, the majority of which were discovered at or after 6 months.15 In the SURPASS registry of the Watchman Flx device cases, the incidence of DRT was low, at 0.2% at 45 days, long-term data are awaited. In the Amulet IDE trial, the DRT incidence was similar between the Amulet and Watchman devices (3.3% versus 4.5%) at 18 months.5 In a dedicated international multicenter registry of 711 LAAO cases with and without DRT that were device-matched and temporally related, the risk factors associated with DRT were hypercoagulability disorder, pericardial effusion, renal insufficiency, implantation depth >10 mm from the pulmonary vein limbus, and non-paroxysmal AF.16
Conclusion
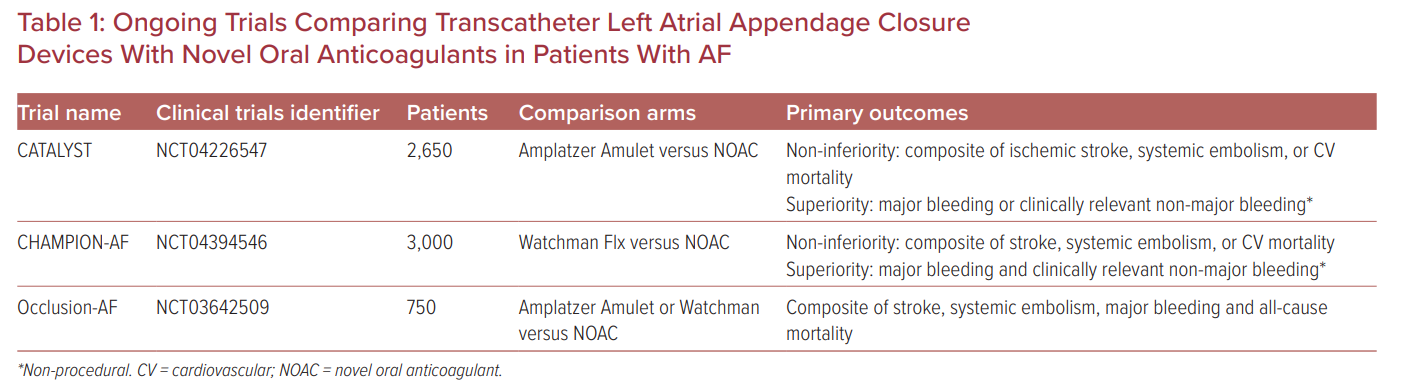
The pivotal trials of LAA closure devices were performed in the warfarin era and prior to the widespread adoption of direct oral anticoagulants (DOAC) over warfarin for stroke prevention in AF patients. It is important to assess the efficacy and safety of transcatheter LAA closure against DOACs in patients with AF. The PRAGUE-17 was a randomized non-inferiority trial comparing percutaneous LAA closure (Watchman or Amulet) with DOACs (95% apixaban) in 402 patients with non-valvular AF and with a history of cardioembolism, clinically-relevant bleeding, or high stroke and bleeding risk.17 At median follow-up of 3.5 years, LAA closure was non-inferior to DOACs for the primary endpoint of cardioembolic events, cardiovascular death, clinically relevant bleeding, or procedure-/device-related complications. LAA closure was superior to DOACs for non-procedural clinically relevant bleeding.17 The results of the three ongoing large randomized controlled trials will provide valuable information on the performance of the current generation of LAA closure devices against DOACs, and will inform which therapies are more effective in preventing stroke and bleeding (Table 1). Further high-quality randomized controlled trials are required to identify optimal anti-thrombotic therapy in terms of antiplatelet versus DOAC therapies with different durations post-transcatheter LAA closure.
The LAA closure procedures are usually performed under general anesthesia and TEE guidance. However, intra-cardiac echocardiography (ICE)-guided LAA closure can also be performed, which does not require the use of general anesthesia. Several studies have shown feasibility of ICE-guided versus TEE-guided LAA closure.18,19 ICE-guided LAA closure certainly has a learning curve; however, with the availability of 4D-ICE catheters, the procedure is becoming relatively simpler. 4D-ICE catheters allow for multiplanar and 3D imaging with less manipulation. Further studies are required to better understand the variability in measurements across modalities (TEE, CT, ICE), and standardizing imaging workflow using ICE modality for LAA closure.
There are several unanswered questions when it comes to DRT and PDLs. Although both Watchman Flx and Amulet devices now have an FDA label to use dual antiplatelet therapy immediately post-implant, the optimal anti-thrombotic regimen post-LAA closure implantation needs more scrutiny. Since the vast majority of the LAA closure procedures are performed in those patients with a prior history of bleeding or who are at risk for bleeding, antithrombotic therapy should be tailored considering competing risks for bleeding and DRT, as well as the presence or absence of significant PDLs. Future studies should focus on identifying the optimal imaging modality to assess PDLs, understanding the associations between the morphology of PDLs with outcomes, and identifying which PDLs need further management and optimal management strategies to tackle them.
The timing of complete endothelization is also variable among patients and devices, and more research is needed to determine whether additional follow-up imaging should be performed in selected patients, as it is relatively uncommon to perform follow-up TEE or cardiac CT after 1 year in US clinical practice. Several reports have been published showing a delay in complete endothelization of the LAA closure device beyond 1 year.20–23 The long-term clinical consequence of incomplete endothelization of the LAA closure device beyond 45 days or 1 year, when the majority of the patients are not on anticoagulation, is unknown.
If transcatheter LAA closure shows reliable long-term efficacy and safety as compared with novel oral anticoagulants, it may become the preferred choice for cardioembolic prophylaxis in the majority of patients with AF. Efforts should continue on innovations and refinement of device technology. Devices that conform to individual LAA anatomy, being completely occlusive and thereby reducing the rates of any PDLs, will be more appealing. The thrombogenicity of the device should be as low as possible, thus limiting the need for potent antithrombotic therapy post-implant. An ideal device should be non-thrombogenic; however, that would require major advancement in the technology.












Comments