Coronary artery disease (CAD) is a leading cause of morbidity and mortality globally, despite advances in medical and preventive therapy. It is estimated that 18.2 million adults in the US have CAD, with 720,000 Americans projected to have a first hospitalization for MI or CAD death this year.1 Treatment of patients with symptomatic CAD includes guideline-directed medical therapy and coronary revascularization procedures, percutaneous coronary intervention (PCI) and coronary bypass grafting (CABG), to reduce adverse clinical events and improve quality of life.2–5
The evolution in PCI technology and technique has improved the risk–benefit ratio and resulted in a greatly expanded population eligible for PCI, including high-risk patients with older age, complex anatomic lesions, and multiple comorbidities that preclude surgical revascularization.6 According to 2020 American Heart Association statistics, PCI is the most common revascularization modality and is applied to patients with increased lesion complexity and comorbidities, with about 50% of all PCI performed in patients ≥65 years of age.1 Recent analyses report numerical doubling of unprotected left main PCI from 2009 to 2016, an increase in PCI for baseline left ventricular (LV) systolic dysfunction from 13% in 2004 to 17% in 2016, and for chronic total occlusion from 0.1% in 2012 to 3.4% in 2016.7–9 Thus, high-risk PCI (HR-PCI) is emerging as a valuable therapeutic modality in the growing patient population referred to as ‘complex high-risk and indicated patients’ (CHIP).
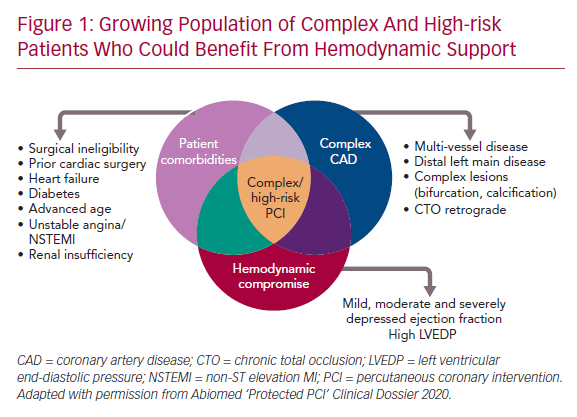
A confluence of characteristics, including complex CAD (multivessel or left main disease and anatomically complex coronary lesions), hemodynamic status (severely depressed LV function), and clinical comorbidities such as advanced age, diabetes, peripheral vascular disease, heart failure, acute coronary syndromes, or previous cardiac surgery define CHIP, although none are absolute (Figure 1). Acknowledging the variable definition of CHIP, many patients with angina refractory to guideline-directed medical therapy or heart failure are candidates for HR-PCI after review by the heart team, per the appropriate use criteria for coronary revascularization.10,11 However, studies suggest underuse of revascularization in >30% of appropriate use criteria patients, which is associated with adverse outcomes.12 While CHIP are least likely to be offered PCI, they are the group most likely to benefit from revascularization.6
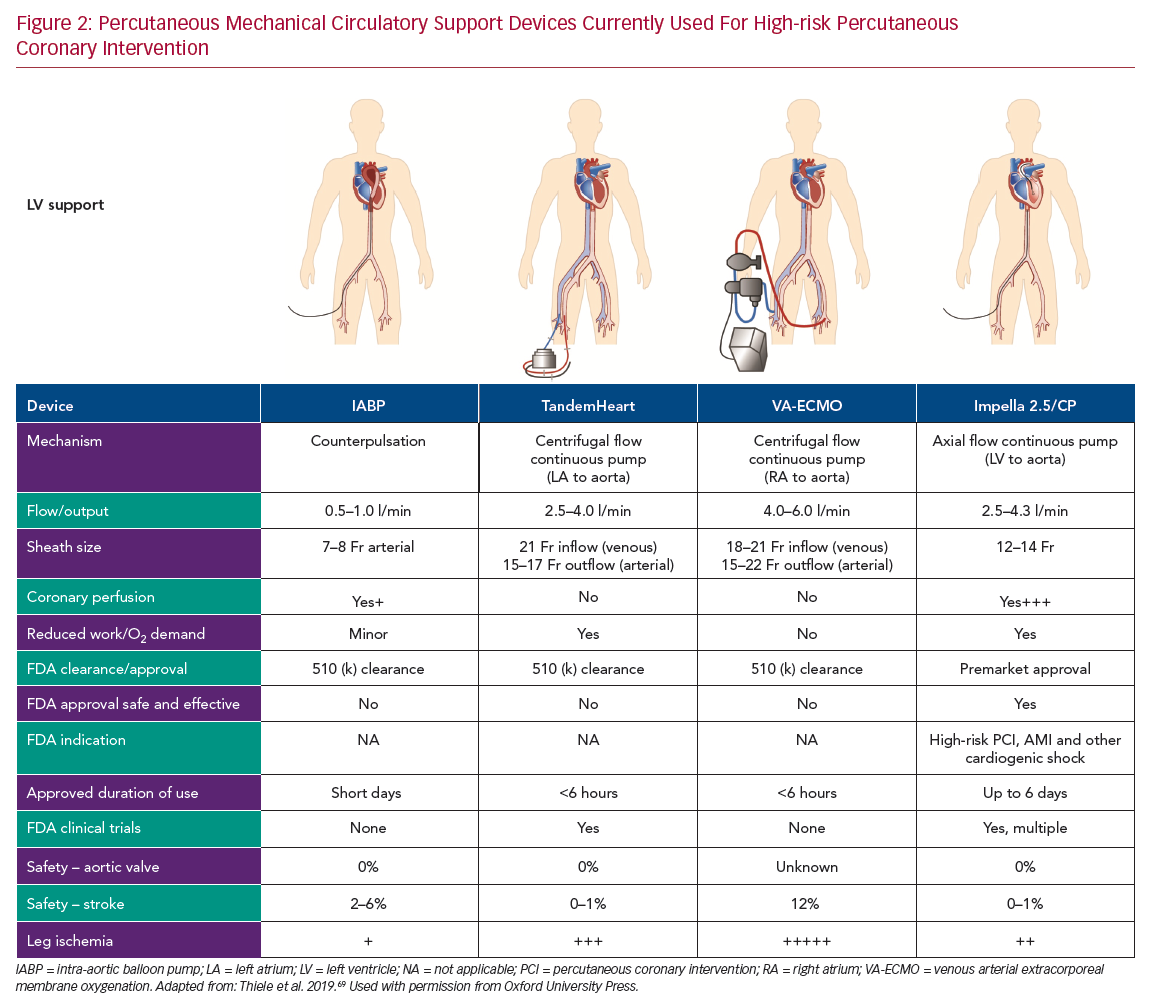
Registry data and retrospective analysis of randomized trials suggest that complete revascularization leads to superior outcomes.13,14 However, given the increased risk of procedural complications induced by multiple balloon inflations and plaque modification procedures, such as atherectomy, CHIP frequently undergo incomplete revascularization or a staged PCI strategy with a higher incidence of adverse clinical outcomes.14–16 Over the last 20 years, multiple percutaneously implanted hemodynamic support devices have become available for use during HR-PCI to prevent hemodynamic collapse and enable complete and optimal revascularization. In this review, we provide an overview of percutaneous hemodynamic support devices currently used in clinical practice for HR-PCI (Figure 2). These include the intra-aortic balloon pump (IABP), centrifugal pumps (TandemHeart [CardiacAssist], venous arterial extracorporeal membrane oxygenation [VA-ECMO]), and micro-axial Impella pumps (Abiomed). Specifically, we discuss the hemodynamic effects of the support devices and clinical evidence of safety and efficacy with a special focus on Impella pumps.
Percutaneous Hemodynamic Support Devices
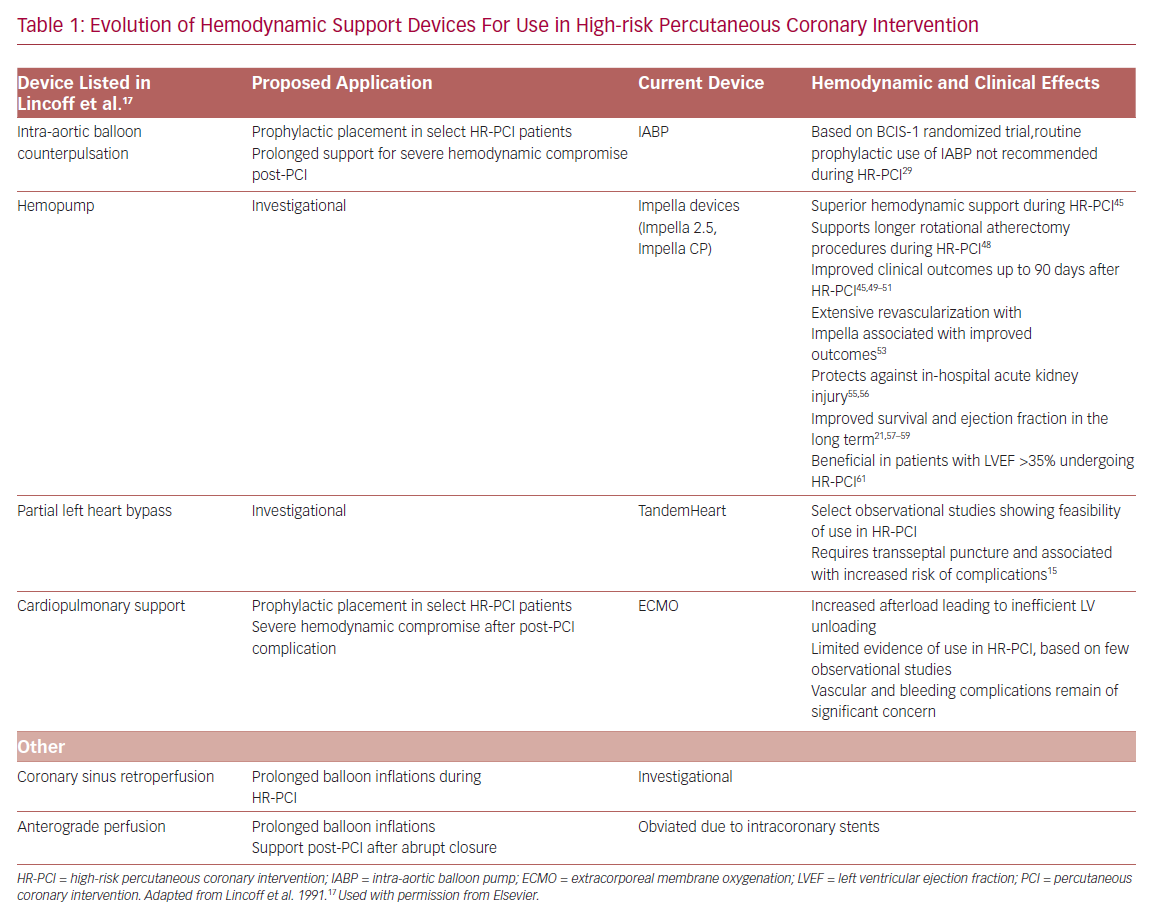
In their seminal 1991 publication, Lincoff et al. listed multiple mechanical support devices and their potential application as an adjunct to HR-PCI.17 It is remarkable that the range of adjunctive PCI tools currently available for disposal in the catheterization lab are mostly iterative developments of the devices proposed previously (Table 1). The goal of hemodynamic support during HR-PCI is to maintain mean arterial pressure to ensure end-organ perfusion and decrease myocardial oxygen demand while maintaining or increasing the cardiac output. In addition, an ideal hemodynamic support device would facilitate complete revascularization in a single setting, aiding LV remodeling and recovery of LV ejection fraction in the long-term. Despite the ability for optimization of hemodynamics, the risks associated with the large-bore access for all these mechanical support devices include bleeding and vascular complications.18–22
Intra-aortic Balloon Pump
The first case report of successful treatment with an IABP was reported in a 45-year-old woman with acute MI with cardiogenic shock in 1968.23 Since then, IABP has evolved as prophylactic support during HR-PCI. IABP provides circulatory support by displacing blood volume in the descending aorta by inflating during diastole and reducing resistance to systolic output through presystolic deflation of the balloon.24 The overall effect of the IABP is to reduce myocardial work and oxygen demand by 10–20% by decreasing the duration of isometric phase of LV contraction.17 Hemodynamically, IABP reduces LV end-diastolic pressure (LVEDP) by up to 30% and systolic pressure by 10%.24 Nonetheless, the IABP only provides a modest increase in cardiac output of 0.5–1 l/min and requires a stable electrical rhythm or pressure tracing for optimal timing and function. Consequently, the use of IABP is of limited hemodynamic benefit in CHIP, particularly those with depressed LV function or contractility.17
Several observational studies have suggested a reduction in mortality and major complications with the elective use of IABP during HR-PCI.25–28 The Balloon Pump-Assisted Coronary Intervention Study (BCIS-1) was the first randomized trial to evaluate the safety and effectiveness of elective IABP use in HR-PCI. A similar incidence of the primary endpoint of major cardiac and cerebrovascular events (MACCE) at hospital discharge (capped at 28 days) was observed among patients undergoing HR-PCI with elective IABP support versus without planned IABP support.29 A post-hoc long-term follow-up study of BCIS-1 suggested a 34% reduction in all-cause mortality with elective IABP use than unsupported PCI, though did not provide any mechanistic explanation of the effect based on LV function and remodeling.30 Romeo et al. performed a meta-analysis including 11 studies and found no correlation of elective IABP use in HR-PCI with a reduction in the risk ratio for in-hospital death or major adverse cardiovascular events (MACE).31
Centrifugal Pumps
TandemHeart
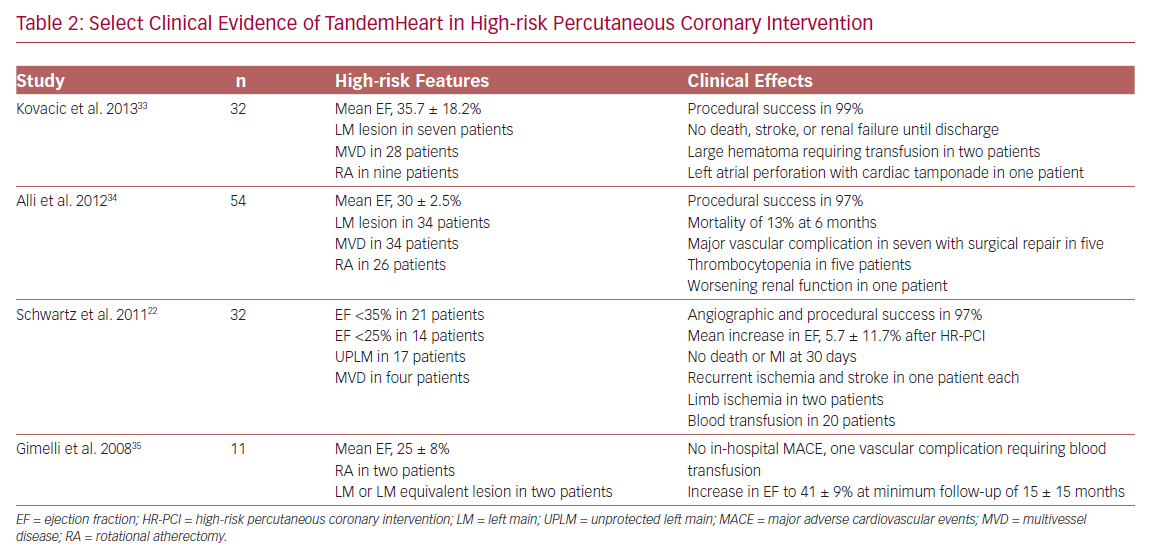
The TandemHeart is an extracorporeal left atrium to femoral artery bypass system. It consists of a 21 Fr venous transseptal inflow cannula containing 14 side holes and a large end hole, a continuous flow centrifugal pump, and a 15–17 Fr arterial outflow cannula.15 The device delivers up to 4 l/min of blood flow and is dependent on left atrium volume and right ventricular (RV) function for optimal function. It is approved for use in cardiogenic shock for up to 14 days, and an oxygenator can be added to the circuit allowing for concomitant circulatory and oxygenation support.32 Hemodynamic effects include a reduction in LV preload and workload, filling pressures, myocardial oxygen demand, and increased arterial blood pressure, and cardiac output. Limited data on the use of TandemHeart for HR-PCI suggest the feasibility and effectiveness of support (Table 2).22,33–35 However, limitations include transseptal puncture and higher complication rates.
Extracorporeal Membrane Oxygenation
ECMO is a portable modification of heart-lung bypass machine that consists of a centrifugal pump, heat exchanger, and membrane oxygenator.18 This device drains venous blood through one or multiple outflow cannula into the external centrifugal pump, where it is sent to the oxygenator for gaseous exchange and the oxygenated blood is returned to the venous (VV) or arterial (VA) circulation through an inflow cannula. While VV-ECMO provides respiratory support, VA-ECMO provides both respiratory and hemodynamic support. VA-ECMO can provide cardiac flow of 4–6 l/min and be used for managing both RV and LV dysfunction. The main indications for ECMO include profound cardiogenic shock with respiratory failure and cardiac arrest.18
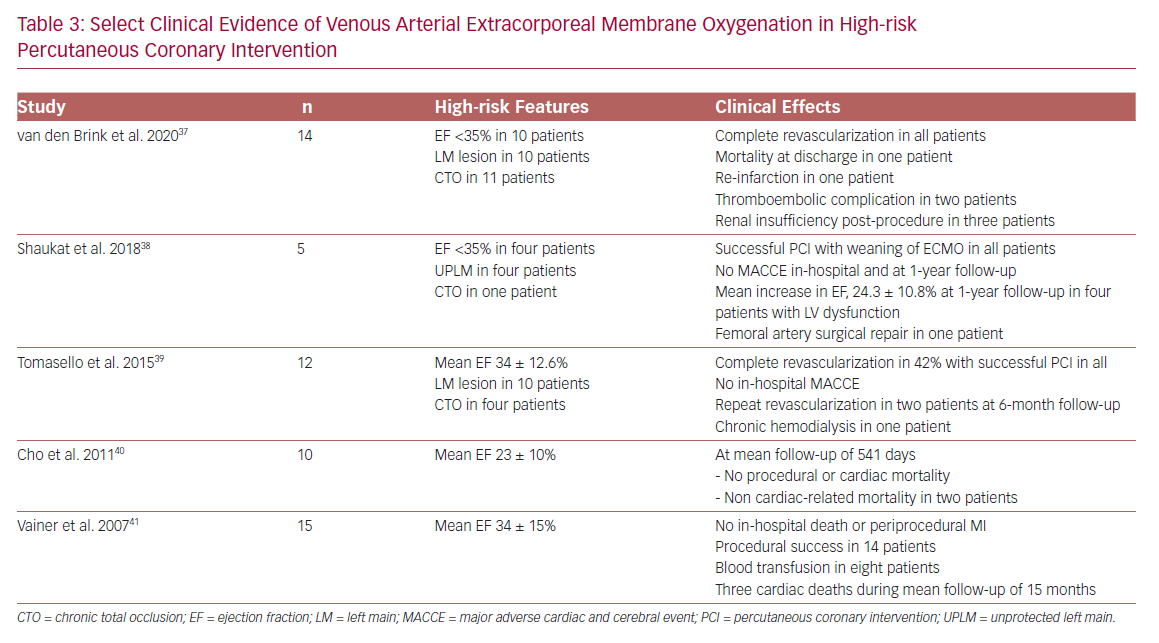
The primary hemodynamic effects of VA-ECMO are decreased preload and increased afterload. The increase in afterload may contribute to LV distention, elevated LVEDP, increased myocardial oxygen demand, and an ultimate decline in myocardial perfusion in patients with significant LV dysfunction.36 Limited data for VA-ECMO use in HR-PCI suggest feasibility,37–41 although vascular and renal complications remain a significant concern (Table 3).
Impella
The Impella is a non-pulsatile micro-axial flow Archimedes screw device that is placed across the aortic valve and designed to pump blood from the LV into the ascending aorta, in sync with the normal physiology. Impella devices (2.5 and CP) are placed percutaneously via peripheral arterial approach, femoral or axillary arteries. Impella 2.5 and CP have motors that are 12 Fr and 14 Fr and provide blood flow rates of 2.5 and 4.3 l/min, respectively. Impella continuously pumps blood directly from the LV, independent of the cardiac cycle, resulting in LV unloading (LV volume dependent).32 With increasing pump flow rate, the LV becomes increasingly unloaded, leading to reduced LVEDP, decreasing LV work, and myocardial oxygen demand. Also, the greater degree of unloading results in increased dissociation of LV peak pressure and aortic pressure, referred to as ventriculoarterial uncoupling.36,42 Impella improves distal coronary pressure and coronary perfusion pressure in the presence of critical stenoses, lessening the ischemic burden.43 The Impella 2.5 pump has been commercially available since 2008, upon receipt of the Food and Drug Administration (FDA) 510 (k) clearance in the US. The Impella 2.5 and Impella CP heart pumps received FDA premarket approval as safe and effective ventricular support devices for HR-PCI, referred to as Protected PCI, in 2015 and 2016, respectively.32
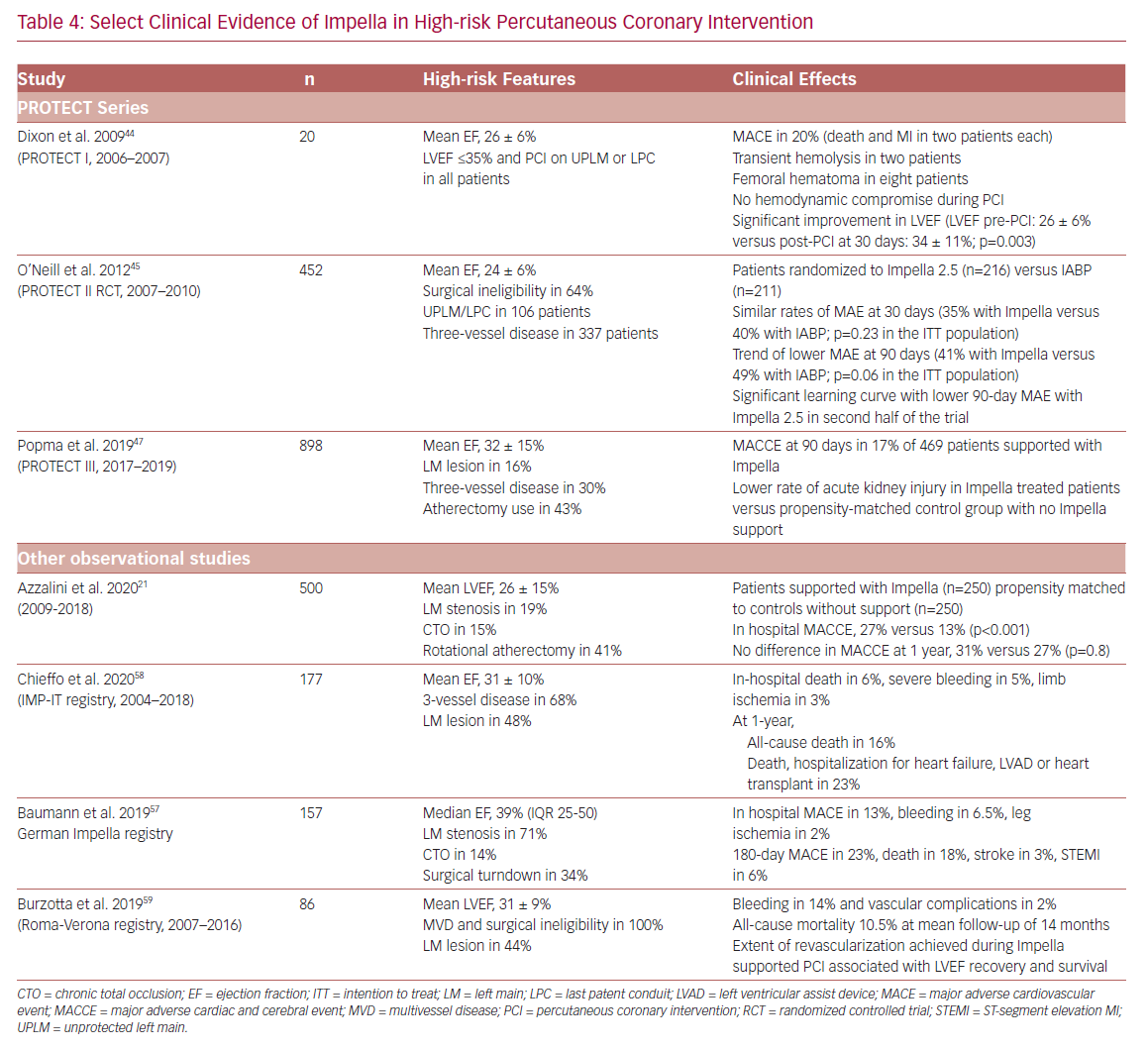
The clinical evidence supporting the safety and effectiveness of Impella support in HR-PCI includes a prospective single-arm feasibility study (Prospective Feasibility Trial Investigating the Use of the IMPELLA RECOVER LP 2.5 System in Patients Undergoing High Risk PCI; PROTECT I), a randomized controlled trial (Prospective, Randomized Clinical Trial of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk PCI; PROTECT II), an FDA post-approval study (PROTECT III), and several observational multicenter registries including the Roma-Verona Registry, the Observational Multicenter Registry of Patients Treated with IMPella Mechanical Circulatory Support Device in ITaly (IMP-IT), and German Impella registry (Table 4).
PROTECT I was a prospective, single-arm, multi-center feasibility study examining the safety and feasibility of Impella 2.5 in HR-PCI.44 Between 2006 and 2007, 20 patients with LV ejection fraction (LVEF) ≤35% undergoing PCI on an unprotected left main lesion or last patent conduit were enrolled. The study showed an excellent safety profile of the device, with MACE at 30 days in 20% of patients (two MIs and two deaths). None of the patients developed hemodynamic compromise during PCI. Also, significant improvement in LVEF was observed with the use of Impella 2.5 during HR-PCI (LVEF pre-PCI: 26 ± 6% versus post-PCI at 30 days: 34 ± 11%; p=0.003). Based on these results, Impella 2.5 received the US FDA 510 (k) clearance in 2008 for partial circulatory support for up to 6 hours during cardiac procedures and led to the pivotal PROTECT II trial.
PROTECT II was a prospective randomized controlled trial comparing hemodynamic support with Impella 2.5 versus IABP in patients undergoing HR-PCI (2007–2010).45 Patients with complex three-vessel disease or unprotected left main and LVEF ≤35% were randomized to an Impella 2.5 (n=216) or IABP (n=211) support. The primary endpoint was a composite of 10 major adverse events (MAE) at discharge or 30 days with a follow-up at 90 days: death, stroke/transient ischemic attack, MI, repeat revascularization, need for cardiac or vascular operation, acute renal dysfunction, cardiopulmonary resuscitation or ventricular arrhythmia requiring cardioversion, increase in aortic insufficiency >1 grade, severe hypotension, and failure to achieve angiographic success. The trial was stopped prematurely, based on an interim review of the primary endpoint, following enrollment of 452 of the planned 654 patients. However, a prespecified subgroup analysis revealed a learning curve with Impella 2.5 during the first half of the trial, leading to underestimation of the potential benefit of Impella at the interim review.45,46
PROTECT III is an ongoing, prospective, FDA post-approval study of Impella-supported HR-PCI patients. Between 2017 and 2019, a total of 898 patients have been enrolled, including 571 supported with Impella CP.47 Compared to Protect II, patients in PROTECT III are older, include more women, and receive more complex procedures.
Effect of Impella Support During High-risk PCI
Superior Hemodynamic Support of Impella 2.5
In Protect II, Impella provided superior hemodynamic support compared to IABP (maximal decrease in cardiac power of 0.04 ± 0.24 W with Impella versus 0.14 ± 0.27 W with IABP; p=0.001).45 Only 6% of Impella patients were discharged from the catheterization lab on the device, compared to 37% of IABP patients. Consequently, the duration of hemodynamic support was longer in the IABP arm than with Impella 2.5 (8.4 ± 21.8 hours versus 1.9 ± 2.7 hours; p<0.001).
Supports Longer Rotational Atherectomy Procedures
Rotational atherectomy (RA) is used for treating complex, heavily calcified lesions and is associated with increased risk of hypotension and periprocedural MI. In PROTECT II, RA was used more frequently and aggressively in the Impella arm with more RA passes per lesion and longer duration of use than IABP.48 This treatment imbalance likely resulted in a higher rate of periprocedural MI (creatine kinase myocardial band [CK-MB] >3 times the upper limit of normal [ULN]) in the Impella group at 30 days (34.4% versus 5%; p=0.014) with no difference in mortality. Notably, the rates of repeat revascularization were lower with Impella at 30 and 90 days.
Short-term Clinical Outcomes with Impella Support During High-risk PCI
Improved Clinical Outcomes up to 90 Days
In PROTECT II, no difference in the composite of MAE was observed between the groups at 30 days (35% with Impella 2.5 versus 40% with IABP; p>0.05). The 90-day MAE was lower in the Impella arm than IABP in the per-protocol comparison (40% versus 51%; p<0.05).45 This difference in MAE was driven by fewer repeat revascularization events with Impella 2.5 at 90 days.
In a post-hoc analysis based on a periprocedural MI definition of CK-MB >8 × ULN, the 90-day MAEs were lower with Impella due to less repeat revascularization and MI.49 The lower 90-day MAE rates with Impella supported PCI was maintained in the subgroup of patients with three-vessel disease and LVEF <30% (40% versus 51%; p<0.05)50 and those <80 years of age (40% versus 52%; p<0.05).51 The lower MAE also led to lower readmission and length of stay costs with Impella 2.5 (5 days versus 7 days and $11,007 versus $21,834; p<0.001), thus being more cost-effective than IABP.52 Consistent improved outcomes with Impella were observed with lower MACCE rates at 90 days in PROTECT III (16.8%) than in the PROTECT II Impella arm (21.9%).47
Extensive Revascularization with Impella Associated with Improved Outcomes
Burke et al. evaluated the benefit of Impella 2.5 versus IABP support as a function of the extent of revascularization.53 More extensive revascularization was associated with improved 90-day MAE compared to limited revascularization. Among patients undergoing extensive revascularization, Impella support was associated with lower 90-day MAE than IABP (32% versus 50%; p<0.05).
Impella Protects Against Acute Kidney Injury
Periprocedural acute kidney injury (AKI) is observed in 4–28% of patients undergoing HR-PCI, depending on the definition of AKI used.45,49,54 Flaherty et al. compared the in-hospital incidence of AKI among 115 patients with LVEF <35% undergoing Impella 2.5 supported PCI versus 115 unsupported matched controls.55 Despite the presence of pre-existing chronic kidney disease and lower LVEF, only 5.2% of Impella-supported patients developed in-hospital AKI versus 27.8% of unsupported controls (p<0.001). Also, post-procedure hemodialysis was needed in only 0.9% of Impella patients versus 6.1% of controls. Consistent results of a lower incidence of AKI than expected based on the Mehran risk score were obtained among 223 patients undergoing HR-PCI supported with Impella 2.5/CP in the global cVAD study (a prospective, multicenter, FDA post-market study).56 The putative mechanism of action includes the maintenance of continuous blood flow during Impella-supported PCI, thus reducing renal hypoperfusion and preventing stagnation of contrast material in the renal tubules.
Long-term Clinical Outcomes With Impella Support After High-risk Percutaneous Coronary Intervention
Improvement in Survival and Ejection Fraction
Multiple registries have reported long-term clinical outcomes following Impella-supported PCI, including the German Impella registry (n=157, 6 months follow-up), IMP-IT registry (n=177, 1-year follow-up), and the Roma-Verona Registry (n=86, mean 14 months follow-up).57–59 A common limitation of all these retrospective analyses includes the lack of a control group (no hemodynamic support or other devices) and ascertainment bias. Also, the comparison of mortality and adverse event rates across these studies is challenging given the variable baseline patient characteristics and the threshold for device usage. Nonetheless, the all-cause mortality at 1-year among patients supported with Impella during HR-PCI were similar at 15.6% in the IMP-IT registry58 and 15.3% in the analysis by Azzalini et al.21
Burzotta et al. investigated the effect of extent of revascularization on LVEF and survival in 86 patients undergoing Impella-supported PCI in the Roma-Verona registry.59 At a mean follow-up of 14 months, the all-cause mortality rate was 10%. In addition, reassessment of LV function at 6 months after HR-PCI demonstrated a 3-fold increase in the number of patients with ejection fraction ≥35% (67% of patients had ejection fraction ≥35% at 6-month follow-up compared to 22% at baseline). Notably, the extent of revascularization was associated with significant improvement in LVEF and survival. These results are consistent with the observations of Daubert et al.60 In the PROTECT II trial, suggesting reverse LV remodeling and an associated improvement in LVEF following hemodynamically supported extensive revascularization in addition to the immediate reversal of the ischemic and hibernating myocardium.
Impella Support Beneficial in Patients with LVEF >35% Undergoing High-risk PCI
Alaswad et al. compared the effects of Impella 2.5/CP support during HR-PCI in 661 patients with LVEF ≤35% versus 230 with LVEF >35% from the cVAD study.61 Notably, patients with LVEF >35% had severe comorbidities and complex angiographic features necessitating Impella support. Despite several high-risk features among those with LVEF >35%, the observed in-hospital mortality was 1.7%, lower than the predicted Society of Thoracic Surgeons (STS) mortality rate of 4.9%. This study suggested that elective Impella use during HR-PCI is safe, feasible, and beneficial among those with complex CAD and LVEF >35% in addition to those with LVEF ≤35%.
Guidelines
The role of hemodynamic support in HR-PCI is only minimally addressed in the guidelines because of the lack of evidence from randomized trials. Currently, the role of Impella in HR-PCI has been addressed in expert consensus documents.62–64 The 2011 guidelines state that elective insertion of an appropriate hemodynamic support device as an adjunct to PCI may be reasonable in carefully selected high-risk patients (Class IIB, level of evidence C).65 The 2010 European Society of Cardiology guidelines suggest that circulatory support should be considered in non-emergent HR-PCI procedures such as left main disease, single remaining patent coronary artery, and complex chronic total occlusions performed by adequately experienced operators at centers that have access to circulatory support and on-site cardiovascular surgery.66 However, no recommendations for specific devices are provided.
Ongoing and Future Studies
Restore EF is an ongoing real-world quality metric study investigating the effects of Impella-protected HR-PCI on the improvement in LVEF at 60–180 days in over 500 patients.67 This multicenter, prospective, single-arm, observational study was initiated in 2019 to capture the intermediate-term clinical outcomes from electronic health records of patients who underwent Impella-supported HR-PCI at up to 30 centers globally.
PROTECT IV is a recently announced on-label randomized trial comparing HR-PCI with Impella CP versus standard of care in patients with LVEF ≤40% and prohibitive risk for CABG.68 The study is currently being designed. It aims to begin enrolling patients in 2021 and will be based on validated best practices with Impella use.
Conclusion
Patients with LV dysfunction, complex CAD, and multiple comorbidities are a growing population often deemed ineligible for surgical revascularization. Hemodynamic support devices act as an adjunct to HR-PCI maintaining hemodynamics, ensuring end-organ perfusion while decreasing myocardial oxygen consumption. While the use of IABP is on the decline based on the failure to show benefit in the BCIS-1 trial, centrifugal pumps such as TandemHeart and VA-ECMO are sparingly used due to increased complications. The safety and efficacy of Impella 2.5 and Impella CP in HR-PCI has been demonstrated in the PROTECT-II trial and multiple real-world studies over the past 12 years. Future randomized controlled trials, such as PROTECT IV, will provide more definitive answers on the role of hemodynamic support during HR-PCI and strengthen guideline recommendations.