Aortic regurgitation (AR) is one of the most prevalent valvular heart disease.1 Untreated patients have a high risk of progressive deterioration, congestive heart failure, and mortality.1,2 Surgical aortic valve replacement (SAVR) is currently the standard treatment for pure AR.1 However, novel treatment methods are needed for patients with high surgical risk. We report a case of congestive heart failure accompanied by cardiorenal syndrome due to severe AR, which was deemed to carry a prohibitive surgical risk and was successfully treated with transcatheter aortic valve replacement (TAVR).
Case Report
An 84-year-old male with a history of hypertension, diabetes, and coronary artery disease was referred to our valve clinic due to worsening symptoms of exertional and resting dyspnea, fatigue, bilateral lower extremity edema, and New York Heart Association functional class IV status during the preceding 6 months.
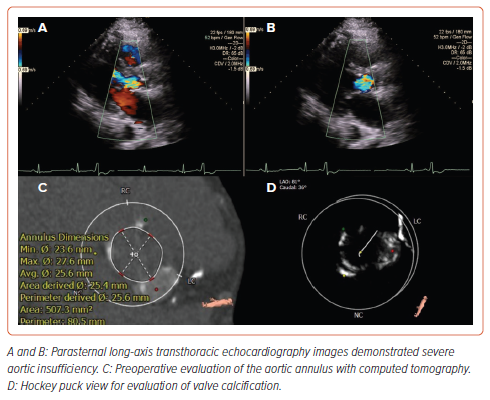
Diuretic resistance remained despite optimal medical therapy and increased diuretic dose (recurrent), leading to recurrent hospitalizations with cardiorenal syndrome and renal failure (serum creatine level at baseline 1.3 mg/dl; follow-up: 3.9 mg/dl). Transthoracic echocardiography revealed slightly lower left ventricular systolic function (left ventricular ejection fraction 50%) with severe aortic regurgitation (vena contracta 0.63 cm; pressure half-time 140 ms, large central regurgitant jet) and no significant aortic stenosis (Figures 1A and 1B). The Society of Thoracic Surgeons Predicted Risk of Mortality was calculated as 9.3%. The patient was deemed at high surgical risk for SAVR by the multidisciplinary heart team. 3D CT imaging indicated an aortic annulus perimeter of 80.5 mm with a maximum diameter of 27.6 mm, a minimum diameter of 23.6 mm, a mean diameter of 25.6 mm, and an area of 507.3 mm². Additionally, some calcification was observed in the aortic root, with an aortic valve calcium score of 1200 Agatston units (Figures 1C and 1D). Coronary heights were 16.1 mm for the left ostium and 19.7 mm for the right ostium.
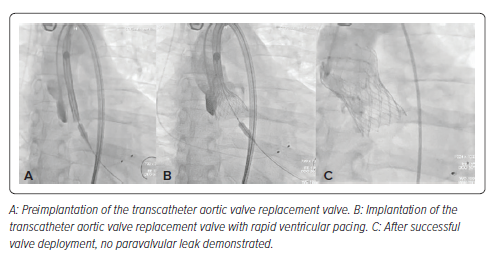
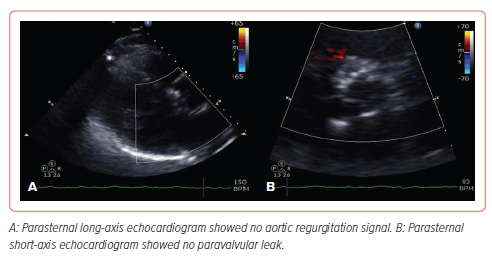
Under moderate conscious sedation, a 34 mm Evolut R (Medtronic) was deployed, facilitated by rapid left ventricular pacing and gradual deployment, resulting in immediate AR resolution without any significant peri-device leak (Figures 2 and 3). To decrease the risk of valvular embolization, the positioning and anchoring of the valve were meticulously confirmed by biplane aortography. The guidewire was not retrieved until after a longer observation period than is standard for TAVR procedures, in case emergency valvular retrieval became necessary. A SAFARI-2 extra support small curve guidewire (Boston Scientific) was selected to maintain stability during valve deployment due to its high curve retention profile. The patient exhibited significant clinical improvement after the procedure, attaining normal renal function within 3 days. At his 6-month follow-up, he had resumed daily activities without limiting symptoms (exertional and resting dyspnea and fatigue) or further hospitalizations.
Discussion
Our case highlights the clinical significance of severe AR, which affects approximately 13% of patients with native valvular heart diseases.1,2 Traditional SAVR remains the standard of care for isolated, severe symptomatic AR.2 However, in patients with high surgical risk, alternative therapeutic approaches are needed. Our case report demonstrates an alternative and feasible approach using a conventional self-expanding TAVR valve for pure AR cases complicated with cardiorenal syndrome and high surgical risk.
Several distinct characteristics of the Evolut R contributed to its success in this case. This self-expanding valve, made with a porcine pericardial material and supra-annular leaflets, offers both durability and easy delivery. The supra-annular design optimizes valve orifice dimensions and leaflet coaptation, ensuring a large effective orifice area.3 The valve is available in various sizes (23 mm, 26 mm, 29 mm, and 34 mm), and its optimal implantation depth, approximately 3 mm below the virtual annular plane, enhances radial forces and optimizes frame apposition, contributing to good results.3
Three primary anatomical factors significantly contribute to challenges faced during TAVR in cases involving pure AR (e.g. valve embolization): an elliptical and enlarged annulus, concurrent dilatation of the aortic root, and a lack of calcification in both the aortic annulus and leaflets.4 Optimal valve size, positioning, and anchoring are paramount in preventing valve embolization. Increased stroke volume, often associated with significant AR, coupled with the challenge of low implantation height due to the absence of fluoroscopic calcific landmarks, poses additional risks.4 We emphasize the importance of prolonged observation before guidewire removal to monitor for potential valve inversion or embolization, which may necessitate balloon recapture maneuvers.
Accurate determination of the prosthetic valve size is critical. Prosthesis/annulus oversizing by ≥15% is associated with a reduction in residual aortic insufficiency and insufficient prosthesis anchoring.4 Oversizing beyond 25% is not recommended due to the risk of annular rupture and conduction system abnormality.5 The Evolut R used in our case was 17% larger than the native aortic annulus.
Precise valvular implantation is essential to mitigate the aforementioned challenges. This can be facilitated by positioning two pigtail catheters in the aortic sinuses, using transesophageal echocardiography guidance, and selecting the correct workhorse wire. The safety of the SAFARI-2 workhorse guidewire for left-sided structural heart interventions has been previously demonstrated.6 This guidewire is used to maintain stability and safety while deploying the valve. Furthermore, rapid ventricular pacing serves as a valuable tool in reducing stroke volume, stabilizing the annulus, and limiting prosthesis motion during deployment. In select cases, preparedness for cardiopulmonary bypass may also be warranted.
A recent study examined optimal anchoring of the TAVR valve in isolated AR based on aortic annulus (AA), left ventricular outflow tract, sinotubular junction (STJ), and thickening leaflet.7 Four possible sites were suggested: inside the left ventricular outflow tract below the AA (−4–0 mm), at the AA, above the AA (0–8 mm), and at the STJ to 40 mm above the AA.7 The STJ may help anchor the ‘crown’ of the prosthetic valve, preventing it from slipping downward. Moreover, the thickening leaflets greatly increase friction between the native valve and the prosthetic valve frame.4,7
Prosthesis choice can significantly influence TAVR outcomes. Newer generation prostheses, especially those designed for native leaflet anchoring, have shown superior performance compared to earlier valves, particularly in cases where significant aortic valve calcification for anchoring is lacking.8 Additionally, self-expanding valves offer repositionability and aortic stabilization. Balloon-expandable valves with prominent outer skirts and the option to oversize significantly present another viable alternative.8
Emerging technologies specifically designed for isolated AR, such as the J-Valve (JC Medical) and Trilogy (JenaValve), have achieved procedural success rates exceeding 90%.9,10
In a recently published, non-randomized, single-arm study, the Trilogy valve demonstrated non-inferiority to SAVR in terms of 1-year follow-up mortality, achieving the performance goal of 25%.10 While the Trilogy is a promising valve technology, its limited global availability and lack of randomized, controlled trial data restricts its use.
Our case report provides insight into the feasibility and efficacy of transfemoral TAVR in patients with isolated AR and a high or prohibitive risk for surgery. Based on our experience, technical precision – including accurate sizing, imaging-guided implantation, and usage of rapid ventricular pacing – plays a pivotal role in achieving favorable outcomes. The choice of prosthesis, particularly newer generation valves and dedicated AR prostheses, can mitigate common complications associated with TAVR. Further research and clinical experience will continue to refine our approach to managing isolated AR and optimizing TAVR outcomes.
Conclusion
This report demonstrates successful treatment of a high surgical risk patient with cardiorenal syndrome due to pure AR using the Evolut R valve system. With meticulous planning and the implementation of our aforementioned recommendations, transfemoral TAVR may be considered an option in the treatment of patients with severe AR and high surgical risk.