Transcatheter aortic valve replacement (TAVR) has become a widely adopted treatment modality for the treatment of severe aortic stenosis. Successful implementation of TAVR requires vascular access that is suitable to accommodate the delivery systems. Advances in sheath and delivery system designs have led to smaller profile devices and expandable sheaths that can be successfully delivered via the transfemoral (TF) approach. The transfemoral TAVR approach, as compared with surgical aortic valve replacement (SAVR), has become the approach of choice for patients due to its ease of use, ability for early mobility, allowance of awake procedures and fast track protocols, and avoidance of surgical incisions. Its superiority as a first-line approach has been confirmed in numerous registries, and also in the PARTNER high- and intermediate-risk studies, in which significantly improved clinical outcomes, such as death and stroke, were demonstrated for the TF approach over transaortic or transapical access.1–4
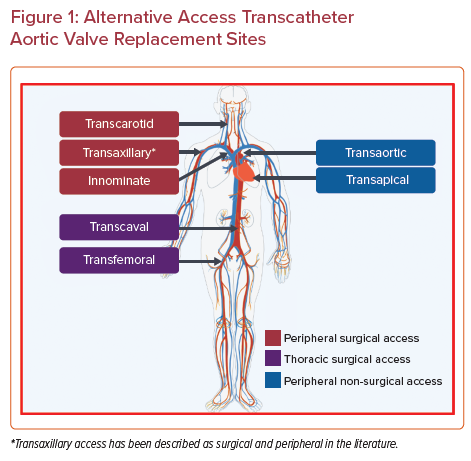
However, it is estimated that one-quarter of the patients undergoing TAVR also have concomitant peripheral arterial disease.5 Despite technological advances, a recent analysis of the Transcatheter Valve Therapy registry showed that 7.6 % of TAVR required non-transfemoral, alternative access.6 Alternative access sites can be broadly categorized into transthoracic and peripheral approaches, facilitated by either surgical or percutaneous techniques. Transthoracic approaches include transapical, transaortic, and subclavian access. Peripheral options include transaxillary, transcarotid, and transcaval access (Figure 1). Current American and European guidelines both recommend TF approach as the access of choice, but do not provide guidance in choosing between various alternative access choices.1,7 In this review, we discuss the technical details and clinical outcomes of various TAVR access approaches for patients with unfavorable transfemoral anatomy.
Shockwave
In patients with calcified iliofemoral vessels with luminal diameters of marginal, but not prohibitive, size, intravascular lithotripsy (Shockwave IVL; Shockwave Medical) is a technique that utilizes electro-hydraulically generated high-speed sonic waves that provide mechanical energy to selectively disrupt vascular calcium. Disruption of intimal and medial calcium in peripheral vessels results in increased compliance without vessel recoil and allows for large bore sheath delivery. IVL balloon catheters are compatible with 6 or 7 Fr sheaths, delivered over 0.014-inch coronary wires, and range from 5.0 mm to 7.0 mm in diameter. This technology has gained Food and Drug Administration approval for use in calcified peripheral arteries in June 2017.8
There are several advantages to IVL. First, IVL distributes sonic pressure waves equally across the lumen diameter and is not subject to guidewire bias. Second, since the IVL balloon is utilized at low pressures, there is a lower risk of vascular injury compared with balloon angioplasty, including dissections and perforations. In fact, the Disrupt PAD III study showed a significantly lower rate of flow-limiting dissections, 1.4% versus 6.8% in IVL compared with balloon angioplasty, and no perforations in the IVL group.9 Finally, IVL theoretically could be expected to reduce the risk of distal atheromatous embolization, but this has not yet been demonstrated by data.
Therefore, IVL is an important treatment option in concomitant aortic stenosis and peripheral arterial disease to maintain the TF approach. Given these advantages of using IVL, its indicated use has been expanded to treat calcified and stenotic vascular anatomy. Disadvantages to its use include its very high cost, which is not reimbursable, and challenging bailout situations if major aortoiliac injuries occur. Iliac artery calcification is the primary reason for abandoning the TF approach to TAVR.10,11 Small (n=42) multicenter registry data have shown promising results with successful TAVR in all patients with treated vessels. It is worthwhile to note that in this group of patients, 80% received 14 Fr sheaths, likely chosen because of the smaller diameter and deliverability in calcified and tortuous anatomies.11 Further data are ongoing and will help provide guidance for the use of IVL in TAVR.
Transthoracic Approaches
During workup for TAVR, assessment of iliofemoral anatomy may show inadequate lumen diameter, excessive tortuosity, and calcification. Luminal diameter must be large enough to accommodate delivery sheath size. Femoral artery calcification has been identified to be predictive of vascular complications in TF TAVR.12 In cases of inadequate luminal diameter and high-risk peripheral anatomy, a transthoracic approach can be considered.
Transapical
The transapical TAVR (TA-TAVR) was the first alternative transthoracic access described. TA-TAVR requires general anesthesia and is most often performed in a surgical hybrid suite. A thorough workup of pulmonary and ventricular function should be undertaken in patients considered for TA-TAVR. The left ventricular apex is approached using an anterolateral mini-thoracotomy in the fifth or sixth intercostal space. Two apical purse-string sutures are placed, allowing for the apex to be punctured, followed by wire, sheath, and device delivery insertion.13,14
Patients who have undergone TA-TAVR have typically been the most comorbid patients; for instance, with the highest EuroScore and Society of Thoracic Surgeons (STS) scores. The TA approach was an integral part of the original PARTNER trial, which included 1,100 patients who underwent TA-TAVR. In PARTNER A, TA-TAVR was associated with higher early mortality and stroke compared with TF-TAVR.15 These results were reassessed by Blackstone et al. using a propensity analysis in an effort to determine whether these results were attributable to the procedural risk of TA-TAVR or to patient selection factors.16 In this analysis, more adverse periprocedural events and a longer recovery time were demonstrated for TA-TAVR, but with equivalent incidence of stroke and lower incidence of significant aortic regurgitation in the TA-TAVR group. Based on these results, the TF approach was recommended as the first access strategy if anatomically feasible.
At the conclusion of the randomized PARTNER trial, the group continued to enroll patients into a non-randomized continued access cohort. The randomized cohort included 104 transapical and 92 surgical aortic valve replacements in the TA group. A total of 975 patients were enrolled in the non-randomized continued access group. The study analyzed the outcomes in the non-randomized TA-TAVR group and compared them with the outcomes of the SAVR randomized group. The groups had no difference in STS-predicted risk of mortality, but the non-randomized TA cohort was older and had a higher incidence of prior cardiovascular interventions. The study showed equivalent 30-day, in-hospital, and 1-year mortality. There was a lower incidence of 30-day or in-house stroke mortality and overall neurological events among the non-randomized TA group compared with patients randomized to SAVR. The favorable outcomes of the TA group may be attributable to patient selection factors in this non-randomized study.16
The evaluation of SAVR versus TA-TAVR in intermediate-risk patients was reported in a subgroup analysis of the PARTNER II trial. The analysis showed no significant difference in death from any cause or stroke between the SAVR and TA-TAVR groups, suggesting no specific advantage of TA-TAVR over SAVR in moderate-risk patients.3 Furukawa et al. compared minimally invasive aortic valve replacement versus transapical TAVR versus transfemoral TAVR in intermediate-risk patients.17 Occurrence of stroke, perioperative MI, and mortality were similar among the three groups. Each group had varying periprocedural complications. Although there was no statistical difference in survival, there was a trend toward worse survival in the TA-TAVR group compared with both the TF-TAVR and SAVR group. The study highlights the importance of carefully evaluating patient characteristics to determine the most appropriate approach for aortic valve replacement.
Transaortic
Transaortic (TAo) is an alternative transthoracic approach available to patients with anatomy unsuitable for transapical, such as chest wall deformity, poor pulmonary function, or decreased left ventricular function. The approach avoids a thoracotomy, decreasing the incidence of pain, and minimizes the effect on respiratory status, which may contribute to the decreased length of hospital stay in this approach compared with TA.18 Avoiding a left ventricular incision decreases the risk of myocardial injury and left apical bleeding.19 Because of these advantages, the TAo approach quickly supplanted the TA approach among TAVR operators. The use of a transaortic approach is limited in patients with heavily or circumferentially calcified ascending aortas.
Access to the ascending aorta is obtained using an upper hemisternotomy or right mini-thoracotomy (in select cases). A pericardial incision is created to expose the ascending aorta. Two pledgetted sutures in a purse string or U-stitch configuration are placed at the selected location to allow for direct needle puncture of the aorta. A soft wire is introduced, the aortic valve is crossed, and a sheath is placed to allow for introduction of the delivery device and valve deployment.20
Dunne et al. performed a systematic review comparing the short-term outcomes of transapical and transaortic approaches for TAVR.21 The review included 60 articles with a study population of 9,961 patients, which included 342 transaortic patients and 9,619 transapical patients. They reported similar baseline characteristics for the two groups. The 30-day mortality in the transaortic group was 7.9%, which was slightly lower compared with 9.7% seen in the transapical group. Statistical significance was not met when evaluating the stroke rate, but a trend toward a lower rate was seen in the transaortic group. The occurrence of conversion to a surgical valve, paravalvular leak, pacemaker requirement, and major bleeding were equivalent in the two groups.
Long-term outcomes comparing the two transthoracic approaches were evaluated by Lardizabal et al.22 This single-institution retrospective evaluation compared the 1-year and longer outcomes of patients who underwent TAo and TA-TAVR. All-cause 30-day mortality was similar in both groups. The long-term, all-cause death at 1 year was higher in the TA-TAVR group, which may be attributable to the higher degree of comorbidity in the TA group, who had a higher median STS score.
Peripheral Access
In patients who are not candidates for standard TF TAVR, guidelines endorse a surgical option to be re-evaluated.23 However, with advancements in TAVR techniques and device profiles, non-femoral peripheral (n-FP) accesses (transcarotid and trans-subclavian/axillary) have arisen as safe and efficacious alternatives with similar outcomes to TF.
The largest experience comparing femoral peripheral (FP) access and n-FP comes from Beurtheret et al. in their multicenter analysis of data from the FRANCE TAVI registry of n-FP procedures performed from 2013 to 2017.23 All 1,613 n-FP cases utilized a surgical approach. The n-FP cohort was a sicker population with higher mean logistic EuroSCORE, and higher rates of peripheral vascular disease and cardiopulmonary comorbidities than the TF counterpart. After propensity score-based matching of patients with FP and n-FP interventions, the groups had similar outcomes at 30 days, with no differences in post-procedural death, access site complications, or stroke. However, patients in the n-FP group experienced a twofold lower rate of major vascular complications and unplanned vascular repairs.
Transcarotid
In patients with challenging iliofemoral anatomy unsuitable for transfemoral TAVR, a transcarotid option can be explored. The patient’s anatomy is evaluated for TAVR workup using CT imaging and Doppler ultrasound. Carotid artery dimensions with a luminal diameter of ≥6 mm are sought, and significant stenosis ≥50% or plaque at high risk of embolization should be ruled out. Careful evaluation for other contraindications to the transcarotid approach should also be assessed, which include subclavian, vertebral, carotid stenoses or occlusion, or aortic arch variants. The patency of the circle of Willis, which is important to provide flow from the contralateral carotid during a transcarotid approach, should also be evaluated using imaging, including cerebral magnetic resonance angiography and transcranial Doppler ultrasound.24
An incision is made along the anterior sternocleidomastoid border above the clavicle to expose the common carotid artery at the level of the omohyoid muscle. The carotid sheath is incised and mobilized, allowing for encircling vessel loops to be placed for distal and proximal control. A micropuncture kit is typically used to enter the artery and exchanged for a 5 Fr sheath. Once the aortic valve is crossed and the delivery wire is in place in the ventricle, the vessel loops are then tightened and the distal common carotid artery may be clamped to prevent distal embolization during delivery sheath placement and valve deployment (depending on surgeon preference). A transverse arteriotomy is typically created to allow for delivery sheath passage. After valve deployment and system removal, the artery is back bled and closed or repaired primarily.24–26
Carotid exposure can be performed under local anesthesia and conscious sedation or general anesthesia. Debry et al. compared outcomes between the two types of anesthesia. They found no difference in 30-day or 1-year mortality, or 1-month clinical efficacy or early safety.27 In their cohort, they found a higher rate of stroke and transient ischemic attacks in the general anesthesia group compared with the local anesthesia group. Ultimately, though, the choice of anesthesia at a given institution will be dependent upon the heart valve team’s experience and expertise.
One cited advantage of the transcarotid approach, as compared with, for instance, a subclavian approach, is the shorter distance to the aortic valve, which allows for favorable device control.28 Safety outcomes are similar to TF-TAVR. Watanabe et al. demonstrated non-inferiority of transcarotid to TF in their retrospective study examining 30-day outcomes of 726 patients.29 There were no significant differences in 30-day mortality (8.4% versus 5.0%) or stroke rate (1.2% versus 2.6%) in the transcarotid versus TF groups, and both had similar favorable outcomes with regard to echocardiographic parameters. There was a trend toward increased major vascular complications in the TF cohort. Fluoroscopy time and radiation exposure were significantly shorter in the transcarotid group.
Overtchouck et al. reported outcomes of 314 patients who underwent transcarotid TAVR who were ineligible for TF TAVR. All cases were performed under general anesthesia and predominantly utilized the left carotid artery. The stroke or transient ischemic attack rate was 1.4%, with a 30-day mortality of 3.2%, similar to rates of the TF approach in PARTNER II.3,24
The transcarotid approach has also been compared with transthoracic approaches. Chamandi et al. considered a multicenter consecutive cohort of patients who required alternative access TAVR. In this cohort, 101 patients underwent transcarotid approaches, while 228 underwent transthoracic approaches. There were similar rates of 30-day mortality, stroke, need for new pacemaker, and major vascular complications between groups, but the transcarotid TAVR group experienced less new-onset AF, bleeding, and acute kidney injury, and had a shorter median length of stay. The results of this cohort suggest a clinical benefit of transcarotid compared with alternative transthoracic access options.30
Allen et al. compared short- and medium-term outcomes in a retrospective study of patients with similar risk profile and STS score undergoing transcarotid versus transapical and transaortic access for TAVR.26 They found a trend toward lower 30-day mortality in the transcarotid group, and significantly improved survival at 2 years in favor of transcarotid access. Additionally, the transcarotid group experienced shorter hospital length of stay, fewer transfusions, and more frequent discharges home than the central access cohorts. There was no difference in stroke rate at 30 days, 2.4% in the transcarotid, 3% in the transaortic, and 2.1% in the transapical group.
Transaxillary/Subclavian
The axillary/subclavian artery is an additional peripheral vessel alternate to the femoral or carotid arteries. The use of the axillary artery for TAVR has traditionally been through a surgical cutdown followed by direct arterial puncture or via a Dacron graft conduit. The axillary artery is approached in the deltopectoral groove lateral to the pectoralis muscle, whereas the subclavian artery requires an infraclavicular incision medial to the pectoralis minor.31 In the direct arterial puncture approach, direct repair of the artery is typically undertaken. The axillary artery is a more fragile vessel than, for instance, the femoral artery; its decreased tensile strength and compressibility can be attributed to its diminutive media layer. This, along with its close anatomical relationship to the brachial plexus, has led many to prefer the surgical approach; however, percutaneous access is also possible and is growing in popularity.32,33
The percutaneous approach is performed using direct puncture in the deltopectoral groove using ultrasound and fluoroscopic guidance with contrast injected via the brachial artery, or by placing a wire from the femoral artery into the axillary artery for guidance with fluoroscopy.31 After valve deployment, the access site is closed using percutaneous closure devices.34 Achieving hemostasis can be challenging at times, as the clavicle prohibits direct pressure application to the artery. When considering the axillary artery for alternative access, vessel size, tortuosity, presence of a patent internal thoracic mammary conduit, angulation of the subclavian to the arch, and the aortic root angle must be evaluated. The left axillary artery is more often used given the more direct course to the aortic annulus, allowing for a larger range of aortic root angles.32
Subclavian/axillary access has also been found to have equivalent outcomes to those of TF in the propensity matched analysis of patients from the CoreValve (Medtronic) US Pivotal Trial Program.35 General anesthesia was used in 99% of the trans-subclavian cohort, and 96% underwent a surgical cutdown; the remainder were performed percutaneously. There was no difference in procedural times, all-cause mortality, major vascular complications, stroke, or bleeding at 30 days and 1 year. Dahle et al. analyzed the frequency of transaxillary TAVR in the Transcatheter Valve Therapy registry report.36 The analysis showed that transaxillary TAVR is the most frequently used alternative access with high procedural success (97.4%) and low vascular complication risk (2.5%). There was a 30-day stroke rate of 6.1%, which is higher than reported rates in the high and intermediate cohorts of the PARTNER trial; the reason for the high event rate is unclear from the data.
At many centers, the transaxillary approach has evolved to be the alternative access option of choice. Kindzelski et al. reported their group’s experience with alternative access from 2006 to 2019, which included 2,446 TAVR patients (342 transthoracic and 56 transaxillary). They found that patients who underwent a transaxillary approach required fewer blood transfusions, less prolonged ventilation, and shorter length of stay compared with transthoracic approaches. Survival and major morbidity were similar in the matched comparisons of the transfemoral and transaxillary approaches. No brachial plexus injuries occurred with transaxillary access.37
Comparison of Carotid and Subclavian Outcomes
A small, single-center, retrospective study found similar safety and efficacy outcomes comparing the transcarotid and trans-subclavian TAVR.38 All but one of the 71 patients evaluated underwent surgical cutdown under general anesthesia. The transcarotid approach had a shorter procedural time, and a trend toward less fluoroscopy time and radiation exposure. In-hospital and 30=day post-procedural outcomes were similar between the groups. There were no differences in mortality, composite major bleeding and vascular complications, perioperative blood transfusion, or need for a postoperative permanent pacemaker at these time points. The 0% and 3% 30-day mortality in the transcarotid and trans-subclavian group, respectively, was substantially lower than in previous studies. The 30-day stroke risk was not statistically different between the groups.
The risk of stroke is of particular concern when accessing the carotid or subclavian/axillary artery. The 2016 study by Mylotte et al. evaluating 96 elderly patients in the French Transcarotid TAVR Registry had an overall 30-day transient ischemic attack/stroke risk of 6.3%.39 A variety of potential mechanisms of stroke during transcarotid TAVR have been proposed: embolization from arterial puncture, access site trauma leading to in situ thrombosis, inadequate contralateral perfusion, and embolization of debris from the calcified aortic valve. While seemingly counterintuitive, it has been suggested that the risk of embolization of debris may actually be reduced with the carotid artery sheath occluding the neck vessel during transcatheter heart valve deployment.
While data suggest equivalent patient outcomes between the two approaches, a few additional factors are worth mentioning. Some particularly favor the right transcarotid for its very direct path to the aortic valve, simplifying TAVR prosthesis deployment. Others favor the left common carotid artery, as its anatomical location minimizes any potential injury or embolization to the innominate artery that feeds the right carotid and vertebral distribution.38 Axillary/subclavian cutdown may be challenging in obese patients. However, other experts prefer trans-subclavian for its close proximity and relatively straight course to the annulus.35 Additionally, there is a theoretical decreased stroke risk with use of the left subclavian, as it only traverses the left vertebral artery territory.31 The selection is often dictated by the vessel with larger size and least tortuosity along with the level of expertise of the surgical team in arterial exposure and handling.
Suprasternal Direct Innominate Artery
A more recent method is the use of the innominate artery with a suprasternal approach, avoiding a sternotomy or thoracotomy for alternative access TAVR. Under general anesthesia, an incision is made at the suprasternal notch, the platysma is divided, and the strap muscles are mobilized, exposing the avascular plane over the trachea. Blunt dissection behind the sternum between the innominate artery and vein, with division of the right sternothyroid muscle, exposes the anterior surface of the innominate artery. Two Prolene purse-string sutures are placed to allow for access and placement of the sheaths and delivery system of the TAVR. Once the valve is deployed, the system is removed, and the purse strings are tied down to obtain hemostasis at the access site.40
The use of the suprasternal approach has been shown to be a safe alternative approach in patients who are not candidates for TF TAVR.41,42 Eudailey et al. retrospectively reviewed patients who underwent suprasternal TAVR. A total of 84 patients underwent suprasternal TAVR, all of whom had technical success with a 30-day survival of 98.8% and no transient ischemic attacks or strokes, with a low incidence of any major bleeding or return to the operating room for bleeding. They found the technique to be safe and reliably reproducible.43
Transcaval
Transcaval access is accomplished through the creation of an aortocaval tract, and makes use of the IVC and retroperitoneal pressure gradient, such that blood from the aorta shunts to the IVC through the tract. The key to planning for transcaval access is identifying a non-calcified aortic segment below the renal vessels and above the aorto-iliac bifurcation that can be stented with a covered graft if a bailout is required, but also close enough to the venous puncture site that can accommodate a 35–40 cm sheath.44,45
A guiding catheter is placed in the IVC with a heavy tip load wire (e.g. Confianza pro 12 or Astato 20; Asahi) on a 0.014–0.035 inch wire converter piggyback catheter that will later facilitate exchange for a 0.035-inch Lunderquist wire. The back end of the venous wire is attached to an electrocautery via a hemostat. The 0.014 inch wire is advanced from the venous side to arterial side as 50-W cutting energy is applied for 1–2 s. Once aortic position is confirmed, the 0.014 inch wire is snared and together advanced to the ascending aorta, and then exchanged for a Lunderquist via a 0.035 inch microcatheter.44,45 Following this, TAVR proceeds in the same stepwise fashion as TF TAVR.
The closure of the transcaval site requires careful maneuvering of the closure device and the TAVR sheath to avoid aortic injury and premature or partial removal of the sheath, because this can result in retroperitoneal bleeding, as the aortic IVC channel is occluded. The choice of closure device depends on the length of the aortocaval tract, with AmplatzerTM muscular ventricle septal defect occluders used for tracts <7mm and Amplatzer duct occluders used for longer tracts. The diameter of the device is selected based upon the size of the sheath used.44 Mild aortocaval fistulas after closure device placement are common; any fistula with more than mild extravasation or hemodynamic compromise requires further investigation.
Early experience from transcaval TAVR studies has shown encouraging results.45 Device access and closure were successfully performed in 98 of 100 patients, with overall inpatient survival of 96% and 30-day survival of 92%. A total of 35% of patients required two or more units of blood transfusion post-procedurally, and overall, 12% of patients were adjudicated as having life-threatening or major bleeding by the VARC-2 criteria. Vascular complications occurred in 13% of patients, and eight patients required covered stents. Further, post hoc multivariate analysis showed a significant increase in bleeding and vascular complications with lower center experience. Aortocaval fistulas were present in 64% of patients immediately after closure device deployment, which was reduced to 36% by 30 days on follow-up with CT and angiography. Four patients required covered stent placement due to ongoing extravasation, intolerable left to right shunt, or closure complications. One-year follow-up of the same cohort showed 71% survival. Additionally, 93% of fistulas were shown to be closed by CT, only one remained patent. There were no cases of occluder device fracture or migration.46
It is evident that transcaval access is more involved compared with shockwave-assisted TF access. Anatomically, transcaval access requires a calcium-free window in the aorta and absence of bilateral iliofemoral disease that are required for snare maneuvers or for bailouts. Moreover, safe transcaval access and closure requires operator experience and a substantial learning curve. Further, transcaval access also requires longer length of stay (2 days versus 4 days, p<0.001) and greater resource utilization. Despite the disadvantages, transcaval access seems to have similar procedural mortality, 30-day readmission rates, and 1-year survival compared with TF access.47 Therefore, avoiding surgical chest access via transcaval access may lead to similar procedural and intermediate-term benefits of TF access, and should thus be reserved when no other non-surgical access is feasible.
Discussion
TAVR has become a common therapeutic option for patients with aortic valve stenosis. Femoral access is the standard, but alternative access is used in patients with unsuitable iliofemoral vessels. Shockwave has been employed in patients with calcified vessels that are found to be adequate in size, allowing for the use of TF-TAVR. Data from the Transcatheter Valve Registry showed that 7.6% of TAVRs are performed using alternative access.
Initially, transthoracic approaches (transapical and direct aortic access) were most common, but the trend has been away from those approaches and toward alternative peripheral access options.48 Registry data has demonstrated a significant difference in the evolution of the distribution of TAVR access sites between 2013 and 2018, with an 11% rise in FP TAVR, a 69% reduction in central access, and a stable frequency of n-FP TAVR.23 Currently in the US, transaxillary access appears to be the preferred alternative access strategy when TF is not feasible.37
In general, the trend away from transthoracic TAVR is supported by data demonstrating superior outcomes of peripheral approaches. For instance, using data from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, Kaneko et al. compared both short- and longer-term outcomes of 3,462 patients undergoing central access (TA and TAo) TAVR, and 3,725 trans-subclavian and transcarotid TAVR from 2015 to 2018.49 They found significantly lower all-cause mortality in the peripheral access group at both 30 days and 1 year, as well as lower rates of blood transfusion, and reduced intensive care unit and hospital lengths of stay. Peripheral access patients suffered a higher rate of stroke at 30 days (5% versus 2.8% <0.001) and at 1 year (7.33% versus 5.54% <0.001).
Across all of the available studies, those in the central access and peripheral cohorts have been a sicker patient population with more advanced comorbidities. Seemingly counterintuitive, despite the less healthy cohort, data from the high- and extreme-risk patients ineligible for TF-TAVR, who underwent transcarotid or trans-subclavian TAVR, have demonstrated equivalent and even improved 30-day and 1-year outcomes when compared with TF.23,29,35,49
There are advantages and disadvantages of the various approaches (Table 1). Although there are no randomized data to support all the alternative access sites, the experiences reported provide available options for a large portion of patients to be candidates for TAVR. The intervention site should be selected by a multidisciplinary heart team based on patient anatomical factors and institutional expertise. It is worthwhile to note that alternative access techniques are typically associated with much higher levels of radiation to the surgical operator than the transfemoral approach.
Finally, it bears specific mention that transthoracic approaches have not been demonstrated to be associated with a specific additional advantage of SAVR in intermediate- and low-risk patients. When a transthoracic alternative access option is considered, careful consideration must be given to the option of conventional aortic valve replacement.