Ventricular arrhythmias (VA), including ventricular tachycardia (VT) and VF, commonly occur in patients with underlying cardiomyopathy. Understanding the differences between the sexes in underlying pathophysiology and anatomy, as well as responses to therapy and outcomes, is critical to providing optimal care for all populations. This article will review sex and gender differences in prevalence, pathophysiology, and treatment, as well as identify areas for future research.
When addressing sex differences that pertain to biological sex at birth or other biological factors (including discussion of chromosomes, sex organs, and endogenous hormonal profiles), we have adopted the SAGER guidelines for sex and gender reporting. As such, we will designate sex differences with the terms ‘female’ and ‘male’. When addressing societal impact factors or studies in which gender was self-reported, we will designate gender differences with the terms ‘women’ and ‘men’.
Epidemiology
Rates of ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM) differ between men and women, with women generally experiencing cardiomyopathy at lower rates than men.1,2 Similarly, men and women experience VA at different rates.
In all patients with a reduced ejection fraction (i.e. with structural heart disease), women are overall significantly less likely than men to experience VA.3 Some studies have shown that men also may have higher rates of severe arrhythmias, and with more events requiring shock and more electrical storms.4
Studies suggest that there are intrinsic sex-related differences in propensity toward VA. In one study of patients with coronary artery disease and ICDs, even after controlling for factors usually associated with VA recurrence, such as inducibility during electrophysiology studies and electrocardiographic factors, men were still twice as likely as women to have an event requiring ICD therapy.4 Another study that adjusted for age, comorbidities, and history of ischemic disease had similar findings.5 In this study, men had a six-fold higher incidence of VT/VF than women after an MI; however, there was no difference in survival between the sexes.5
There is a well-understood relationship between myocardial ischemia and arrhythmia. However, this relationship appears to differ between men and women. Multiple studies in patients with coronary artery disease have shown that women have lower rates of inducible sustained VA than men.4–7 In patients with documented VAs, women are more likely to have NICM, and men are more likely to have ICM.3,8 In the MADIT-CRT trial of patients with ICM, women were 49% less likely to experience VA than men.3 This study also showed that the 3-year probability of VT/VF or death in patients who received an ICD was significantly lower in women.
In NICM, the relationship between sex and rates of arrhythmia is less clear. The MADIT-CRT trial showed that there was no significant difference in rates of VT/VF or death among patients with NICM with ICDs, but the cumulative incidence of only VAs over 4 years was significantly lower in women.3 However, a multivariate analysis by Saxena et al. showed there was a lower risk of VA events in women that was even more pronounced in the NICM population.9
Despite associations between structural heart disease and VA, no structural disease is identified in 10% of patients referred for evaluation of VT.10
Pathophysiology
Sex and Gender Differences in Ventricular Electrophysiology
Differences in ventricular electrophysiologic properties between men and women have been extensively studied. Women have longer QT intervals, ventricular action potential durations (VAPD), and ventricular effective refractory periods (VERP), factors that contribute to an increased risk of torsades de pointes (TdP).11–13 Men, in contrast, have higher incidences of VA in Brugada syndrome and heart failure.11,14–16 These data are primarily derived from animal studies, with a few human studies, and sex hormones appear to account for most of the observed differences.
Female sex is known to be an independent risk factor for development of TdP in congenital long QT syndromes and acquired long QT syndromes; adult women have a 10–20 ms longer QTc interval than men.14,17 Multiple studies have shown that this difference is observed only after puberty, which implicates sex hormones in the development of QT interval prolongation.14,18–22
Mechanistically, sex hormones appear to influence ventricular repolarization through both expression of ion channel subunits and ion channel function.11–13,23 Human and animal studies examining ion channel distribution suggest that female ventricles exhibit less K+ ion channel subunit expression (including hERG, minK, Kir2.3, Kv1.4, KChIP2, SUR2, and Kir6.2) as well as increased divergence of L-type calcium current that account for the increases in VAPD, VERP, and QT intervals.11,12,17,23
Furthermore, hormonal differences between males and females have been shown to directly influence VAPD and thus affect the QT interval and risk of TdP.12 Animal models have demonstrated that testosterone increases the outward potassium currents (Ikr and Iks), the transient outward current (Ito), and the inward rectifier current (Ik1), and shortens VAPD.11,14,24 Conversely, estrogen has been shown to lengthen VAPD by inhibiting Ikr, thereby exerting a pro-arrhythmogenic effect in women.14,18,25 Human studies have also shown that men with hypogonadism secondary to androgen deprivation therapy were more likely to have QT interval prolongation and a higher incidence of TdP, and this effect is reversed by administration of dihydrotestosterone.14,26,27
These sex differences in electrophysiologic properties are not limited to VA occurrence in females. While females are generally at higher risk of long-QT associated arrhythmias, males are more likely to present with VA in Brugada syndrome as well as spontaneous sustained VA in heart failure.11,14 Animal studies suggest that the Ito current density of the right ventricular epicardium is significantly higher in males than in females due to the effects of testosterone, thus increasing the presence of the Brugada-type EKG pattern and arrhythmia formation.11,14,16
Additionally, examination of human myocardial tissue has revealed that sarcoplasmic reticulum calcium leak is higher in males than females and increases the delayed after-depolarizations associated with intracellular calcium overload.28 This finding translates to a higher proportion of observed arrhythmic myocytes and sustained VA in males compared to females with heart failure.28
Sex-related Differences in Ventricular Remodeling
There are also differences in cardiac remodeling between male and female individuals. Human and animal studies show that the remodeling process is overall more favorable in female than in male subjects, particularly in pre-menopausal females. In response to aging, pressure and volume overload, ischemia, and heart failure, women experience greater preservation of cardiac weight, volume, and myocyte number, less maladaptive ventricular dilatation or hypertrophy, and lower rates of apoptosis and fibrosis.29–34
In addition to cardiac remodeling, ion channels themselves may undergo adaptive alterations, which may account for differences in arrhythmia between females and males.35,36 Scarred or damaged myocardial tissue may cause regulatory differences in essential gap junction protein, connexin 43, and Ica, leading to calcium overload and downregulation of potassium currents, thus prolonging ventricular repolarization and creating an adaptive response to improve overall contractility.35–38 Many of these cardioprotective adaptations are more pronounced in females before menopause, suggesting sex hormones play an important role in the remodeling process.
Mouse models have demonstrated antihypertrophic effects of estrogen on ventricular myocytes, leading to a reduction in left ventricular hypertrophy.39–41 Additionally, human and mouse studies support the role of estrogen in protecting against myocardial necrosis and cellular hypoxia through enhanced protein kinase activation and expression of anti-apoptotic gene products.33,41,42
The differences in cardioprotective adaptations between pre-menopausal females and post-menopausal females or males may also be due to the effects of testosterone. Animal and human studies of post-menopausal females have demonstrated positive correlations between testosterone levels and hypertension, decreased HDL levels, impaired vascular reactivity, and cardiac hypertrophy.34,43,44 In mouse models, estrogen has prevented maladaptive cardiac remodeling while testosterone has been shown to impair myocardial healing and exacerbate cardiac dysfunction and chronic structural changes.29,34
It is known that increased necrosis and fibrosis in the myocardium may cause structural abnormalities and reentrant pathways predisposing to VA.45–47 Additionally, left ventricular hypertrophy has been shown to be an independent risk factor for sudden cardiac death and increases susceptibility to VA.45,48–50 The cardioprotective effects of estrogen on cardiac remodeling and delayed deposition of cardiac fibrosis and apoptosis may decrease the occurrence of VA in females compared to males, at least in the pre-menopausal period.
However, sex-specific studies investigating VA in individuals with structural adaptations are scarce.51,52 An observational cohort study examining sex differences in patients with inducible VA reported lower rates in females than males, but the analysis included only nine female patients.51,53
Other studies report sex-specific differences in the origin of VA (right, left, or biventricular), but have not found differences in scar extent or distribution based on cardiac MRI analysis.54,55
Future studies analyzing VA through a sex-specific manner in patients with structural cardiac changes are needed to draw conclusions regarding larger patient populations.
Management
Long-term management of VA is multifaceted and includes treatment of the underlying cause, ICD placement, ablation, and antiarrhythmic drugs.
Antiarrhythmic Therapy
Much of the sex-specific literature regarding antiarrhythmic drug therapy for VA is focused on the adverse effects of antiarrhythmic drugs among men and women. This is especially true among the class III antiarrhythmics.
As discussed, women have longer QT intervals than men at baseline, and thus tend to have more QT prolongation due to the potassium channel blockade of class III antiarrhythmics. A small study examining cardiac repolarization following IV sotalol administration found that women had longer QT intervals than men at any concentration level of sotalol.56
Additionally, a cohort study examining 845 patients initiated on sotalol found that female sex was associated with QT prolongation and a significant predictor of sotalol discontinuation.57 A meta-analysis of 22 multinational trials of patients treated with sotalol for both ventricular and atrial arrhythmias found that women treated with sotalol were up to three times more likely than men to develop TdP.58
Although TdP in the general population is rarely associated with amiodarone use, it has still been associated with increased proarrhythmic effects in women compared to men. A meta-analysis of 332 patients, 70% of whom were female, found that women were twice as likely as men to develop TdP.59,60 In addition to TdP, the FRACTAL trial examining amiodarone use in patients with AF found a significant increase in bradyarrhythmia requiring pacemaker insertion in women compared to men.61
ICD
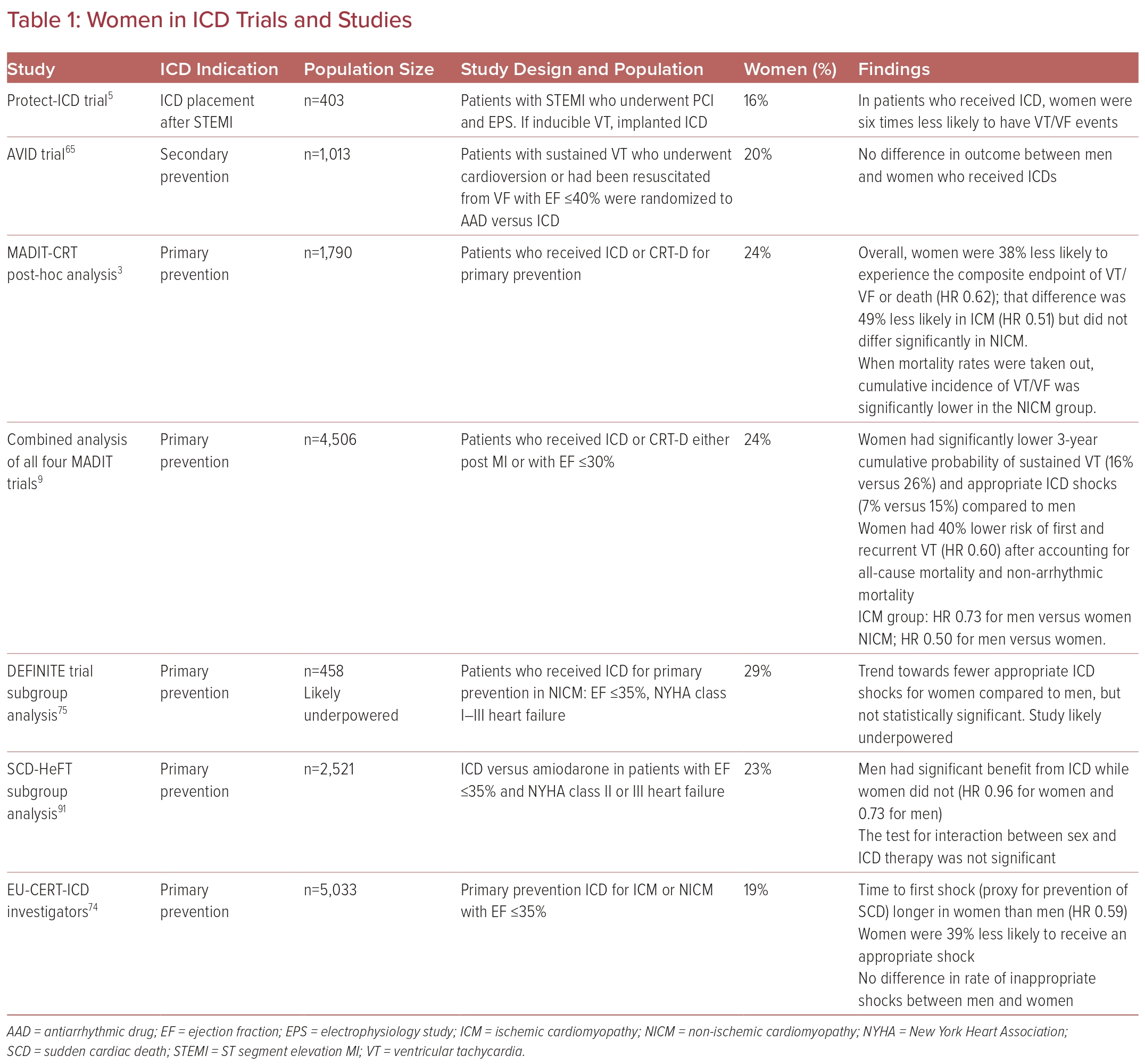
ICD placement is indicated for selected patients for both primary and secondary prevention of sudden cardiac death from VA. Multiple population-based studies suggest that sudden cardiac death rates are lower in women than in men.62–64 However, women have been underrepresented in the majority of large trials investigating ICD use, comprising 14–30% of study populations.6,8,65–71 Thus, data are limited, and the majority come from registries and meta-analyses. Most studies have shown that there does not seem to be a difference in rates of implantation between men and women, at least after referral to an electrophysiologist has been made.6,15,65,72,73
Some studies have suggested that women may not benefit from ICD placement as much as men, although many of the earlier studies for ICD placement did not analyze differences between the sexes, nor did they have enough power for subgroup analyses because of small sample sizes.7,65–71
The benefits of ICD implantation have been defined in literature as both improved mortality and appropriate ICD shock delivery.
In multiple studies, women have been found to have lower rates of appropriate shock or appropriate antiarrhythmic therapy (i.e. anti-tachycardia pacing or shock) as well as a longer time to appropriate shock, suggesting lower rates of prevented sudden cardiac death even when adjusted for comorbidities.5,15,68,74,75 The same studies found no differences in inappropriate shocks between men and women. A recent subgroup analysis of the MADIT-CRT trial corroborated this lower risk of events requiring therapy and also found that rates were even lower in women with nonischemic cardiomyopathy.9
Despite suggestions that women benefit less than men from ICD, they do benefit overall: one study found lower mortality rates in women with an ICD compared to those without (HR 0.79) and this benefit was similar in magnitude to that observed in men in the same study (0.73).76
In addition to benefiting less from ICD implantation, women seem to experience higher rates of adverse events related to devices. Women have higher rates of procedural complications, such as myocardial perforation and pneumothorax as well as later complications including pocket infections, incisional infections, lead revision, and electrical storm.15,74,77,78 Fortunately, there does not appear to be a difference in rates of inappropriate shock. It is hypothesized that reasons for higher complication rates include technical challenges associated with differences in anatomy and cardiomyopathy disease processes (Table 1).
Ablation
Catheter ablation is an effective therapy for reducing the recurrence of VA. Studies suggest that gender differences may exist regarding rates, success, and complications of ablation; however, like ICD trials, data are limited by the lack of representation of women in the major trials on ablation. Women made up between 6.5% and 20% of patients studied in most major trials of VT ablation.79–86 Consequently, many subgroup analyses are inadequately powered to identify gender differences and it is difficult to apply the results of these large studies to women. Larger studies including more women are needed to better elucidate the true relationship between gender and outcomes of ablation.
Some of this difference in representation between men and women in trials likely stems from lower rates of ischemic heart disease in younger women and, therefore, lower rates of clinically significant VT, as mentioned previously.2 In one registry study, women had higher rates of VT recurrence within 1 year following ablation compared with men despite being younger, having fewer medical comorbidities, and having a higher average left ventricular ejection fraction. When broken down by ICM and NICM, women with ICM still had a higher likelihood of recurrence while rates between men and women with NICM were similar.87 The authors of this study hypothesize that women may need a more aggressive approach to ablation, because they had shorter ablation times and higher rates of inducible VT at the end of ablation despite similar periprocedural characteristics and mapping. Inducible VT at the end of ablation has been shown to predict VT recurrence in other studies as well.88
A contrasting study, however, found no difference in recurrence rates between men and women, regardless of whether structural heart disease was present.89 Any differences in success or recurrence are likely not explained by structural differences, as one study found that there were no significant differences in arrhythmogenic substrate between men and women with VT, including scar percentage, scar volume, scar transmurality, and scar distribution.55
Data are also conflicting for complication rates. One study found that sex was not a predictor of periprocedural hemodynamic compensation while another study, which also included atrial ablations, showed that female sex was an independent predictor of periprocedural complications as a whole.79,90 The inclusion of atrial ablation and a composite endpoint in the study by Hosseini et al., as well as the smaller sample sizes in the other studies, could potentially explain these discrepancies.90
Conclusions
Individuals among different sexes and genders differ regarding the prevalence and management of VA. While the discrepancies may be related to differences in type and prevalence of structural heart disease, there may be other factors, including sex hormones and unelucidated mechanisms that warrant consideration.
Additionally, women have been underrepresented in many of the trials evaluating management of VA, so larger trials with greater representation of women are needed to better define best practices for management in this population.