Fibromuscular dysplasia (FMD) is a non-atherosclerotic, non-inflammatory vascular disease, which primarily affects women. Despite once being considered a rare disease, this often-underdiagnosed disease may, in fact, affect up to 1–4% of all adult women.1 Much of what we know about FMD has come from national and international registry data. Currently, clinical registries such as the United States Registry for FMD and the European/International FMD Registry and Initiative are enrolling affected individuals to gain a better understanding of this disease.
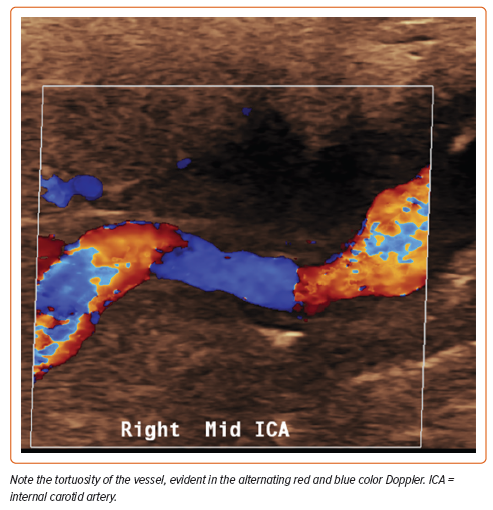
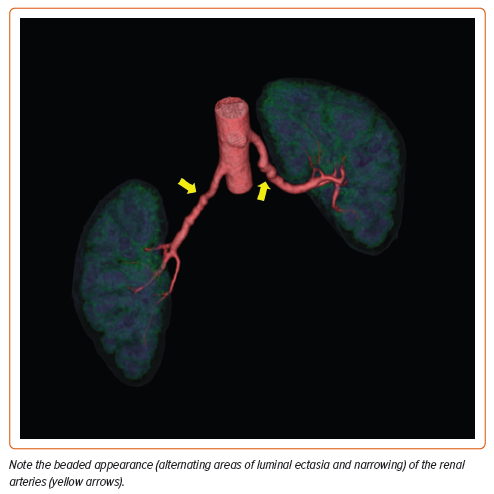
The mean age at diagnosis is 55.7 years, although patients may be affected at any age.2 This non-inflammatory arteriopathy involves primarily medium-sized vessels but may include small and even large vascular beds.3 Typical imaging findings may include ‘beading’ of the arteries (multifocal disease), focal stenotic lesions, or, more rarely, a combination of both. Multifocal FMD is the most prevalent type of FMD and is associated with arterial beading, arterial tortuosity, aneurysms, and dissections (Figures 1 and 2). The vessels most commonly affected are the carotid and renal arteries, but any vessel can be involved (Figure 3), and up to 55% of affected patients have disease in more than one vascular bed.4 Men comprise 10% or fewer of the FMD patient population and may present differently than women. The great majority of affected men have renal involvement (up to 90%) and mesenteric involvement occurs more frequently in men than in women.5
Clinical manifestations vary among affected individuals. Common symptoms include hypertension, headache, pulsatile tinnitus, and dizziness.2 Men are more likely to have serious manifestations, including aneurysm and dissection.5 Previous registry data show that a minority of patients are asymptomatic at the time of diagnosis.2 As time goes on and awareness of this disorder increases, the percentage of patients diagnosed incidentally is likely to increase as well.
Definition
FMD was defined in the 2014 European consensus document as an idiopathic, segmental, non-atherosclerotic and non-inflammatory disease of the musculature of the arterial wall, leading to stenosis.6 The disease affects small- to medium-size arteries, and diagnosis should be made only after exclusion of other causes of stenosis or aneurysmal disease.
Clinical Presentation
According to the US Registry for FMD, the majority of patients diagnosed with FMD present with at least one clinical symptom and only 5.6% are truly asymptomatic.2 The clinical course can vary from incidental findings to severe or life-threatening lesions, and depends on a number of factors: primarily, the distribution of vascular bed involvement and lesion severity.1,2,7,8 The most common manifestation of renal artery FMD is hypertension, whereas patients with carotid or vertebral FMD may present with headache, pulsatile tinnitus, transient ischemic attack, neck pain, dissection, subarachnoid hemorrhage, or stroke.1,2,9,10 Although considered a distinct entity, FMD shares many features with more well-known connective tissue diseases, such as Marfan syndrome, Loeys–Dietz syndrome, and vascular Ehlers–Danlos syndrome. Moderately severe myopia, high palate, dental crowding, and early onset arthritis are prevalent features in patients with FMD.11 Other studies have also noted connective tissue features, such as hip laxity, small joint hyperextension, scoliosis, and pes planus, in patients with FMD.12 Despite the overlap of some features with connective tissue disorders, hypertension and headache (migraine type) remain the most common presenting symptoms of FMD in both men and women.
Diagnosis and Diagnostic Testing
FMD is an arterial disease that consists of hyperplasia of the intimal and/ or medial layers of predominantly medium-sized muscular arteries.12 Histopathological studies of the arterial layer involved, in combination with angiographic arterial appearance, have informed the FMD classifications used for diagnosing the disease today. The three main histological types identified are intimal, medial, and adventitial disease.13,14 Adventitial disease only accounts for the sporadic case of FMD.14 Intimal disease, now considered focal FMD, consists of concentric thickening of the intimal layer of the artery with preservation of the medial and adventitial layer integrity. On angiography, focal FMD can be diagnosed when a focal concentric or tubular stenosis of the vessel is identified. Medial fibroplasia, now multifocal FMD, is characterized by alternating areas of compromised medial layer and thickened fibromuscular ridges caused by collagen deposition replacing arterial muscle.13,14 Accounting for almost 85% of FMD cases, the alternating lesions of medial hyperplasia and vessel stenosis constitute the characteristic ‘string of beads’ appearance of multifocal FMD noted on angiographic imaging.12,15
The differential diagnosis for FMD is broad because there are numerous conditions that can cause hypertension and share imaging features with those of FMD. Although there are still no formal diagnostic criteria for FMD, the First International Consensus of FMD outlines suggestions for when to consider FMD as a leading diagnosis. FMD should be considered for any patient, especially women, under the age of 30 years old who presents with hypertension or any patient with accelerated, malignant, drug-resistant, or severe hypertension.4 Physical exam findings, such as abdominal, iliac, or carotid vascular bruits, should also raise suspicion for FMD in the absence of known atherosclerotic disease or risk factors. The mainstay of FMD diagnosis is evidence of vascular bed involvement on imaging with non-invasive imaging modalities.4
CT Angiography and Magnetic Resonance Angiography
The international consensus on FMD recommends CT angiography (CTA) as the first-line imaging modality in patients with suspected FMD due to its superior spatial resolution and visualization of small calcifications.4 Magnetic resonance angiography (MRA) may be conducted if CTA is nonconclusive or contraindicated.1,4,6,8,9 Both are non-invasive and have good sensitivity and specificity in renal artery FMD detection while being relatively affordable and available.1,4,6,9
Doppler/Vascular Duplex Ultrasound
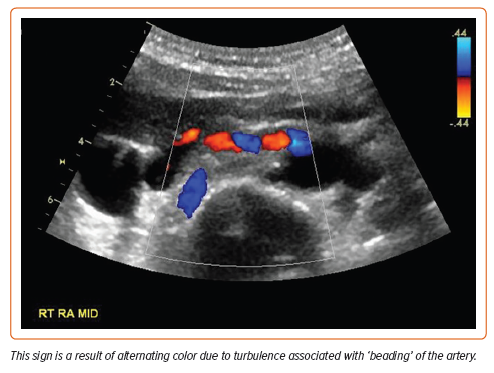
Duplex ultrasound is a widely accessible, non-invasive, non-irradiating, and easily repeated imaging technique that can provide information about kidney size, hemodynamics of stenosis, and associated renal disease. Defining characteristics of FMD on ultrasound include the ‘candy cane sign’: alternating colors representing turbulent blood flow in areas of arterial stenosis (Figure 4). However, ultrasound is time-consuming, highly operator dependent, and less sensitive than CTA or MRA at detecting FMD and atherosclerotic artery lesions.6 Results can also be suboptimal in obese patients or when breath-holding is difficult or impossible. It can be an appropriate first-line screening technique or a surveillance modality after endovascular intervention, but overall it is recommended only as a first diagnostic procedure for renal FMD in specialized centers with highly trained operators of duplex ultrasound.1,4,6
Catheter-based Angiography
Catheter-based angiography remains the gold standard imaging technique for diagnosing and evaluating the location and morphology of FMD.1,4,6,8,9 However, this is an invasive procedure and, despite being the only imaging modality that can accurately identify changes in FMD and aneurysm formation, and dissection of branch vessels, it does not allow for accurate estimation of the severity and hemodynamic significance of renal artery stenosis.1,4,6,9 Thus, the international consensus does not recommend its use in routine clinical practice for diagnosis or surveillance.4 Catheter angiography is indicated only when the findings are suspected to affect the patient’s management by necessitating angioplasty in significant translesional pressure gradients or in scenarios in which clinical suspicion persists despite negative CT or MRA findings.4,8
Pathogenesis
The pathogenesis of FMD continues to be poorly understood. Studies indicate that a combination of environmental, hormonal, genetic, and mechanical factors contribute to disease development.
While the molecular basis is still being investigated, the most common environmental and hormonal factors that have been thought to be associated with FMD include smoking and exposure to endogenous or exogenous female hormones. Smoking has already been established as a predictor in the development of multiple vascular diseases and has been shown to be a risk factor and disease modifier of FMD. In a study in 2013, active smoking was strongly associated with a younger age of hypertension onset and FMD.16,17 More recently, among a cohort of FMD patients, aneurysms and symptoms of claudication were found to be more prevalent in FMD patients who were also smokers.18 It has also been postulated that increased levels of estrogen predispose to the development of FMD, although the exact mechanism remains unclear given that high estrogen states such as pregnancy or the use of oral contraceptives and exogenous female hormones do not predispose women to FMD or FMD-associated complications.1,4
There is also an abundance of studies on the underlying genetic basis and the effects of molecular pathways on the development of FMD. One study showed that a common variant on chromosome 6 in the phosphatase and actin regulator 1 (PHACTR1) gene increases the risk of FMD by approximately 40%.19 Recently, the COL5A1 pathogenic variant c.1540G>A, p.(Gly514Ser) has been identified as the first monogenic factor for multifocal FMD and was found to be associated with vascular manifestations such as arterial aneurysms, dissections, and tortuosity.20 Transforming growth factor (TGF)-β pathways have been investigated as potential pathogenic factors in the development of FMD as a systemic disease. A small cohort of FMD patients were found to have increased secretion of TGF-β1 and TGF-β2 by dermal fibroblast cell lines and elevated plasma levels of TGF-β1, TGF-β2, and other inflammatory markers relative to age- and gender-matched controls.12 Abnormal TGF-β activity may enhance collagen production, which decreases the arterial wall elasticity and predisposes patients to arterial complications.21 Limited data on further association exist and there is still much to be studied regarding the complex genetic patterns of inheritance of FMD.
Management
Management of FMD is dependent on vascular bed involvement, severity, associated complications of aneurysms and dissections, and consideration of symptoms. Counseling, personalized and routine surveillance measures, medical therapy and, less commonly, surgical intervention comprise the mainstays of FMD management. All patients with FMD should have at least one head-to-pelvis imaging to determine vascular bed involvement and to ascertain whether there are any unruptured aneurysms.4
Counseling is often the first step in treating patients with FMD. Smoking has been associated with a higher prevalence of aneurysms in patients with FMD as well as an increased number of adverse events and an increased need for vascular intervention.18 Therefore, counseling on smoking cessation is paramount. Patients with FMD, especially those who have had a dissection, are often counseled to avoid lifting heavy weights and to avoid activities associated with increased risk of dissections such as chiropractic neck manipulation or roller coasters.22
Medical therapy typically entails anti-hypertensive agents and antiplatelet therapy. Given that the intra-arterial webs that characterize FMD may serve as niduses for platelet aggregation, many patients with multifocal FMD are most commonly started on daily aspirin 81 mg in an effort to protect against thrombotic and thromboembolic complications.23 Given that hypertension is one of the most common presenting signs of FMD, most patients are also started on an anti-hypertensive regimen, with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers as the preferred medication.24
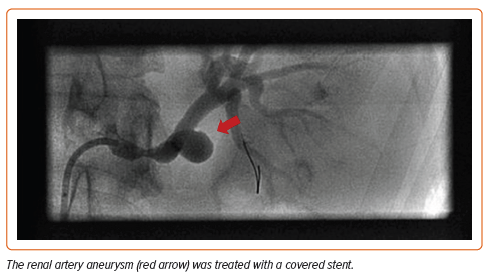
Dissections and aneurysms are common complications of FMD, occurring in more than 40% of patients with FMD.23 For patients who have had spontaneous coronary artery dissection (SCAD), β-blockers are added for proposed protective effects although the use of β-blockers in SCAD management is contested.25,26 Conservative treatment in patients with SCAD is favored because it has improved clinical outcomes compared with the implementation of percutaneous coronary intervention in SCAD patients.27 In many patients who have experienced a SCAD, untreated SCAD segments have been shown to have undergone spontaneous complete vessel wall healing.27 The general consensus is that the treatment of dissections and aneurysms in the setting of FMD should be no different than that of the general population with these vascular pathologies.
Last, adjunct medical therapy with symptomatic and preventative agents is often used to address the concomitant symptoms of migraine and other headache types in FMD patients. Pulsatile tinnitus, a common symptom in those with FMD, can sometimes be managed with sound therapy and cognitive behavioral therapy.28
The question of whether family members of patients with FMD should be screened for the disease is a frequent one. Fewer than 10% of patients in the United States FMD Registry report having a family member with FMD.2 At this time, it is not recommended to screen asymptomatic family members of patients with FMD. For family members who are symptomatic, shared decision-making between the family member and a provider is recommended to determine whether a workup is warranted. As more evidence emerges for genetic associations, genetic testing and counseling will be likely to be used to screen family members for FMD and facilitate discussions for family planning in the future.
Sex-specific Considerations
The Role of Estrogen
Estrogen has been implicated in a number of vascular complications. As mentioned above, it is thought that estrogen, both the endogenous production and exogenous use, is associated with the development of FMD, FMD-related complications, and the occurrence of SCAD.
Reasons for the predominance of FMD and SCAD in women are still being investigated. A pilot study examining specimens from FMD patients undergoing nephrectomy showed that progesterone receptor expression was higher in them than in specimens from controls without FMD who underwent nephrectomy for other, non-vascular causes.29 Through various mechanisms, estrogen has been posited to structurally weaken arterial walls and thus may predispose susceptible patients to arterial complications in situations of hemodynamic change and high stress, a reason why FMD may predispose patients to SCAD.21 Given that it has been posited that sex hormones, through the use of oral contraceptive pills or hormone replacement therapy, might be contributing to the disease process, many patients are counseled to stop these medications once diagnosed with FMD.
Spontaneous Coronary Artery Dissection Association
SCAD is one of the most common etiologies for myocardial infarctions among women, especially during pregnancy and in the post-partum period, and has been shown to be associated with FMD.30
Similar to FMD, it is hypothesized that sex hormones may play a role in SCAD pathophysiology, given that approximately 90% of patients with SCAD are female.21 The high progesterone and estrogen levels during pregnancy and the extreme flux of hormone levels peripartum, paired with the hemodynamic changes that occur during pregnancy and the stresses of labor, are thought to contribute to the higher prevalence of SCAD, as suggested by the up to 43% of cases of pregnancy-related acute coronary syndrome attributed to SCAD.21,30,31 Likewise, patients on hormone therapy seem to have higher SCAD recurrence.32
Interestingly, men who have had a SCAD were found to be significantly younger than women who have had a SCAD, had more cardiovascular risk factors, and had angiographic evidence of intimal tears than their female counterparts.33,34 Additionally, a higher percentage of men with SCAD reported a triggering physical stressor, compared with more women with SCAD reporting a triggering emotional stressor.34–36 While it is understandable that a higher percentage of women who have had a SCAD have FMD due to more women having FMD, it is notable that about one-third to half of men with SCAD were found to have FMD, a prevalence much higher than in the general male population.34–36 This suggests a pathophysiological association between FMD and SCAD. Despite these observed gender differences, most studies examining gender differences in patients with SCAD reported no difference in major cardiovascular outcomes.34–36
At one point it was found that up to 86% of patients with SCAD have evidence of FMD, but more recent studies show that approximately 55% of SCAD patients had FMD.31,37 Furthermore, extracoronary FMD was shown to be one of the independent predictors of major adverse cardiovascular events in patients who experienced a SCAD.31 Therefore it is recommended that all patients who have experienced a SCAD event also undergo one-time head to pelvis imaging to assess for FMD.
Although FMD is commonly found in SCAD patients, the converse has not been shown to be true. FMD is associated with higher arterial tortuosity, which may be a predisposition to arterial dissection, but only approximately 3% of patients in the US FMD Registry have had a SCAD event.4,23
Management of Fibromuscular Dysplasia in Pregnancy
There are limited data on the recommendations for patients with FMD who wish to become pregnant or who are already pregnant. However, patients with FMD who become pregnant are considered to be at high risk and should be followed by appropriate high-risk obstetricians or maternal fetal medicine physicians. Patients with renal FMD are at higher risk for gestational hypertension and hypertensive complications such as preeclampsia and preterm delivery. Additionally, SCAD has been shown to account for more than 40% of pregnancy-associated MIs, and the presence of a peripartum SCAD was also recently shown to be associated with increased major adverse cardiovascular events at 3 years.31,38 Careful monitoring and discussions on delivery options such as caesarean section or assistance with the second phase of labor should be discussed, especially for those with uncontrolled hypertension and those with a prior history of cervical artery dissection or SCAD.4,39–42
Fibromuscular Dysplasia in Men
Although FMD most commonly occurs in middle-aged women, it can also affect men, and may present differently. Men are more likely than women to present with renal artery FMD and thus present with increased manifestations of renal artery involvement, such as abdominal/flank pain (43.8% versus 14.3%), azotemia or renal insufficiency (9.1% versus 2.2%), and renal infarction (42.9% versus 4.3%).5 Women are significantly more likely to present with signs and symptoms of carotid and/or vertebral artery involvement, with higher rates of pulsatile tinnitus (35.7% versus 9.1%), cervical bruit (26.8% versus 4.5%), and neck pain (28.6% versus 13.3%) than men.5 The most clinically significant difference is that men with FMD have a higher frequency of arterial dissection and aneurysms than women with FMD, especially if men are current or former smokers.5,8
Future Directions
More investigation into the genetic and environmental causes of FMD as well as diagnosis and treatment are necessary and ongoing. Up to 7% of FMD patients have first- or second-degree relatives with the disease, and, as noted above, a genetic locus associated with FMD has been found in the PHACTR1 gene.2,19 Current ongoing studies of the genetics of FMD include the DEFINE-FMD study, which is studying FMD as well as SCAD and cervical artery dissection.
Imaging for detection and risk stratification is also being investigated. A 2003 study demonstrated evidence of abnormalities in non-affected arteries when imaged with high-resolution echo-tracking systems.43 This study found a ‘triple signal’: an additional layer between the intima and media thought to represent medial hyperplasia. Findings support the hypothesis that FMD is a systemic vasculopathy. Ultra-high-resolution ultrasound is being investigated in similar ways: evaluating radial wall thickness and other abnormalities in the vessel wall in patients with FMD and SCAD.44,45
Ultimately, further research and clinical investigation is necessary to clarify optimal diagnosis and treatment practices for FMD. Research is also vital to gain a better understanding of the environmental and genetic impacts on this disease. Current ongoing registries such as the US Registry for Fibromuscular Dysplasia and the European/International FMD Registry and Initiative are gathering data on thousands of patients. Data collection and analysis drawn from these are already contributing important findings, and will continue to advance our understanding of this disease.