Vast disparities in cardiovascular morbidity and mortality exist, which partly stem from the underrepresentation of minoritized individuals in clinical research and clinical trials. These disparities encompass sociodemographic factors, such as race, ethnicity, and income status. Another significant source of disparity is biological sex, which is often misconstrued as gender in cardiovascular research. Biological sex or sex assigned at birth is a construct based on anatomical and physiological traits, comprising genetic constitution, hormonal expression, and external genitalia.
Gender, on the other hand, is a multidimensional construct linking gender identity (an individual’s internal identity), gender expression (how they express their identity to others through behavior and appearance), and cultural expectations about social status, characteristics, and behavior that are associated with sex traits.1 Gender-diverse individuals constitute 0.6% of the adult population and approximately 2% of the high-school-aged population in the US.2,3 However, most cardiovascular research has conflated the concepts of sex and gender and defined each as a binary variable, undermining the existence of gender-diverse individuals in clinical research trials.2,3
The cardiovascular health of SGM individuals has remained out of focus in the larger context of cardiovascular guidelines, including the American Heart Association’s Life’s Essential 8, and has been myopically reduced to gender-affirming care, specifically hormone replacement therapy.4,5 This is again due to underrepresentation of this population in cardiovascular research, evidenced by the lack of inclusion of gender-diverse individuals in most studies examining the social determinants of health (SDOH) of cardiovascular disease (CVD).6 Such systemic exclusion impedes the ability to understand the effect of these SDOH on cardiovascular health outcomes which exacerbates the minority stress experienced by SGM individuals.6,7
Equating gender and biological sex prevents us from understanding the complex interaction between gender affirmation and minority stressors, which include both external factors, such as gender-based structural stigma, rejection, violence, and gender non-affirmation, and internal factors including internalized transphobia leading to psychological trauma, depression, and suicidal ideation. Unsurprisingly, these stressors have a significant bearing on the cardiovascular health of SGM people.6–8 An important first step to understanding the effect of these SDOH is prioritizing the collection of data for these two independent concepts (sex and gender) separately. Unfortunately, SOGIE data collection and utilization has been inadequate despite federal mandates.9 Therefore, this paper attempts to raise awareness on the distinction between sex and gender and the need for including both concepts in cardiovascular research.
Sex as a Biologic Variable in Cardiovascular Research
Sex refers to the biological and physical attributes that distinguish male from female, including reproductive organs, gene expression, hormone function, and chromosomes. However, gender is a social and cultural construct that encompasses roles, behaviors, and expectations associated with being of a certain gender.10 Biological sex and gender are related concepts, but they are not mutually exclusive or inclusive. Traditional terms indicating sex (female and male) and gender (by non-inclusive convention, women and men) are often used interchangeably in research studies, which does not allow sex and gender to be accounted for as separate variables. Sex is recognized as a biological variable in cardiovascular health, because there are critical differences in how male and female patients experience and manifest CVDs.11–14 Hormonal influences, genetic factors, and lifestyle choices contribute to these distinctions, emphasizing the importance of considering sex-specific differences in cardiovascular research, prevention, and treatment strategies.15,16 Research has shown that presenting symptoms, risk factors, and disease prevalence varies between the sexes.17 Not only are patient presentations varied, but so is treatment approach and provider bias, when treating similar disease processes in patients of different sexes.18 By excluding sex as a variable in medical research, we miss the opportunity to address knowledge gaps and biases about sex-specific outcomes that affect diagnosis and treatment.
Historically, studies have included mostly male participants, as female patients of childbearing age were often excluded from clinical trials citing safety reasons. This exclusion has led to a limited understanding of how cardiovascular conditions manifest in female patients, and as a result, medical research and care models have been built around male physiology.14 This underrepresentation has had implications on diagnosis, treatment, and overall healthcare for female patients as differences in the manifestation of CVDs have not been accounted for. Sex-based disparities in the diagnosis of acute coronary syndrome (ACS) have been attributed to differences in anatomy, as females have smaller epicardial coronary arteries and lower plaque burden which makes them more susceptible to microvascular CVD.19–21 These factors have been shown to contribute to the differences in syndromic presentations of ACS in female patients compared to male patients. Females may also present with lower cardiac troponin levels, and in the absence of sex-specific thresholds, a diagnosis of ACS may be significantly delayed or missed altogether.21
Female patients hospitalized for ACS have higher mortality rates and readmission rates, and have fewer diagnostic and therapeutic procedures performed during their evaluations than male patients.22–26 Females are also more likely to suffer from heart failure, which has been attributed to the delayed diagnosis of hypertension and ischemic heart disease.27 At hospital discharge, female patients are less likely to be prescribed disease-modifying medications, including antihypertensives and statins, when compared to males.28 The underrepresentation of female patients in preclinical and clinical research has created huge healthcare gaps and disparities in cardiovascular care and outcomes.
Recently, there has been an increased demand from the medical community to ensure more diverse populations are included in cardiovascular research. In 2015, the National Institutes of Health (NIH) mandated the reporting of sex as a biological variable in NIH-funded research. This policy stated, “consideration of sex may be critical to the interpretation, validation, and generalizability of research findings.”11 This was an initiative that was supported by the broader scientific community as many scientific journals began emphasizing the importance of reporting sex as a variable and the possible contribution to treatments and outcomes. Despite this, SGM individuals are yet to be included in large, clinical cardiovascular trials.29
Sex is now recognized implicitly as an important factor in clinical research; however, more work is needed to standardize the way sex and gender are reported and elucidate the way these characteristics function independently and together to influence health and healthcare. Preclinical research studies that incorporate both sexes are crucial to informing the translation of research from basic scientific discovery to drug development and testing of therapeutics.13
Despite the widespread recognition of the NIH’s mandate, research has shown that there were no statistically significant changes in inclusion, analysis, or reporting by sex, compared with studies that predated this mandate, and also showed the studies that recorded sex did not use these data to analyze results.29 Sex is a biological variable that influences differences in screening, diagnosis, provider bias, treatments, and interventions as it pertains to CVD; however, cardiovascular clinical trials are not designed to include or consider this key information. The first step in studying sex-specific outcomes is incorporating research questions and study designs that prioritize a diverse population of participants. Sex as an independent variable can and should be considered on every level of basic and clinical research.
Gender as a Biologic Variable in Cardiovascular Research
Gender Identity/Expression
The term ‘gender medicine’ was first introduced in the late 1990s.30 It is defined as the study of how diseases differ between men and women in terms of prevention, clinical manifestation, diagnostic and therapeutic interventions, prognosis, psychosocial effects, and interactions with the healthcare team. The WHO defines gender medicine as the study of how biological (sex-based), socio-economic, and cultural (gender-based) differences influence an individual’s health.31 Despite these definitions, use of gender in cardiovascular research has been fraught with challenges, due to a general lack of knowledge surrounding the nuances of sex and gender. Furthermore, due to binary labels of sex and gender, gender minority/diverse patients have also not been adequately described in large-scale cardiovascular trials despite the knowledge that SGM groups have been shown to be at increased risk for CVD.32
The very concept of gender is constantly evolving and is therefore quite complex. It has been suggested that gender comprises at least three distinct interrelated components: our physical bodies: how we experience them, and how others interact with them; our gender identity: our internal sense of male, female, a blend of both, or neither; and our gender expression: how we present our gender and how society interacts with the gender we present.33 It is also important to recognize that a person’s gender identity may be the same (cisgender) or different (transgender) from their biological sex. The gender minority stress model has demonstrated that there are numerous factors influencing CVD in gender minority patients.8 Data displaying the relationship between gender minority stress in the SGM population and CVD are limited; however, stressors linked to CVD are well established among heterosexual males and females. Depression is a well-recognized risk factor for CVD that can worsen outcomes with regard to ischemic heart disease and stroke.34,35 Female patients are twice as likely as male patients to develop depression during their lifetime, placing them at increased risk for cardiac events.35,36 Among SGM patients, those who identify as transgender and gender diverse have higher rates of mental health conditions, including depression, suicidality, and anxiety, compared to the general population.35 Among transgender and gender-diverse individuals, one of the greatest factors in the development of mental health conditions is the impact of minority stress.
Distal stressors include gender-based victimization, gender-based rejection, and non-affirmation of their gender identity. Chronic exposure to these stressors leads to ‘proximal’ stressors that include negative expectations for future events, nondisclosure of gender identity, and internalized transphobia.36 This internalization of negative societal attitudes toward transgender and gender-diverse individuals contributes to the higher risk of mental health conditions, increased tobacco and substance abuse, and decreased physical activity, and influence the development of CVD. Given the higher risks of mental health conditions among this subset within the SGM patient population, understanding the correlation between mental health and CVD risk in this population is warranted. As we are on the cusp of understanding the complex relationships between gender and cardiovascular research, there is now an opportunity to understand the role between ischemic heart disease and mental health conditions in SGM people. Results from this type of research could include cardiovascular-based therapeutic interventions to reduce CVD for not only transgender and gender-diverse individuals, but also all SGM individuals.
Despite the increased risk of CVD among SGM patients, this population remains an understudied group in cardiovascular research.37 To date, most literature consists of retrospective cohort studies that are limited by small sample size, lack of control groups, and the conflated reports of sex and gender.38 Through the appropriate use of gender terminology, larger studies are now becoming available to study traditional risk factors (e.g. tobacco use) between cisgender and SGM individuals. For example, data from the PATH study, a large, nationally representative US study, were used to examine tobacco use among SGM populations.39 SGM people were three times more likely to use each of the nicotine/tobacco products (cigarettes, e-cigarettes, and cigars) than their cisgender peers. SGM smokers were more likely to report ‘internal factors’ (stressors and lower quality-of-life self-ratings) compared to cisgender individuals, and both factors were independently related to an increased likelihood of tobacco use.40,41 In contrast to their cisgender peers, SGM individuals are more likely to experience stress and stigma, as it relates to their minority status and SDOH, thus making this population more likely to use tobacco. This study highlights just one example of why it is important to reframe our traditional understanding of CV risks such as tobacco use, which can only be achieved by ensuring we are engaging in inclusive data collection among all individuals including SGM individuals.
In addition to tobacco use, polysubstance abuse, classified as the use of any two substances concurrently, is more common among SGM youth compared to heterosexual youth.42,43 Consistent with gender minority stress models, polysubstance use increased when stressors, such as victimization and discrimination, were experienced among SGM patients. Thus, screening for substance use in transgender and gender-diverse youth remains crucial as there is evidence of a graded relationship that exists between the increasing number of substances abused and the higher likelihood of early-onset atherosclerotic CVD.42,43
Gender Affirmation
Gender affirmation or gender transitioning is a process of outwardly expressing an individual’s internal sense of gender rather than the gender assigned to them at birth. Gender affirmation is divided into multiple elements, including social affirmation (coming out, choosing names and pronouns, and being accepted and supported by friends and family), legal affirmation (legal name change, change of gender marker on identification forms), medical affirmation (e.g. hormones and surgery), and psychological affirmation (acceptance of one’s own gender identity and expression).44
SGM adolescents report a greater incidence of mental health issues such as depression, anxiety, increased risk of suicidal behaviors, and increased substance use, including alcohol, tobacco, and other illicit drugs.45 SGM adolescents also have numerous barriers to healthcare access due to fear of stigma, discrimination, lack of provider knowledge of transgender care, and SDOH (homelessness, lack of medical insurance). Lifetime exposure to these gender minority stressors contributes to poor physical health, which is associated with the development of cardiovascular risk factors (e.g. obesity and hypertension) that lead to increased risk of CVD throughout the lifespan of the transgender patient.4,46
Most cardiovascular research on gender affirmation is related to medical affirmation, including gender-affirming hormone therapy (GAHT) and its effect on cardiovascular risk and disease. For transgender women, feminizing hormone therapy includes estrogens, antiandrogens, and progesterone. Estrogen is a primary sex hormone and is responsible for developing female secondary sex characteristics, such as vocal pitch, breast, and female-specific fat deposit pattern. Antiandrogen therapy decreases endogenous testosterone production and reduces male secondary sex characteristics.
In a 2016 meta-analysis, oral estrogen-based treatment was shown to have a statistically significant association with increases in serum triglyceride levels, increases in LDL cholesterol levels, and decreases in HDL cholesterol levels at 24 months of therapy. However, evidence to support these data remains limited.46 Interestingly, with regard to blood pressure, a small retrospective study of transgender women with an average follow-up of 30 months while on estrogen/antiandrogen GAHT showed a significant decrease in systolic blood pressure of 6 mmHg. However, the authors suspected it was more likely from a reduction of testosterone than increased estradiol.47 A recent larger cohort study of 2,517 transgender women from the Netherlands with a mean follow-up of 9 years shows that transgender women using GAHT had a higher incidence of MI compared to cisgender women, and a higher incidence of strokes and venous thromboembolic events compared to cisgender women and men.48
Regarding transgender men, testosterone is the primary hormone in masculinization therapy. It aids in the development of male secondary sex characteristics, including deepening of the voice, broadening of the shoulders, and male-pattern hair growth. In addition, testosterone therapy also causes increased overall body mass, muscle mass, bone mass, acne, sexual desire, and erythrocyte production. Masculinizing GAHT has been shown to have a statistically significant association with increases in serum triglyceride levels and LDL cholesterol and decreases in HDL cholesterol at 24 months of therapy.46,49,50 Other studies have shown a statistically significant increase in systolic and diastolic blood pressure by 4 and 3 mmHg, respectively.48 There are mixed results on the relationship between masculinizing GAHT and CVD. A large cross-sectional study using data from the Behavioral Risk Factor Surveillance System showed that transgender men had a twofold increase in the rate of MI compared to cisgender men and a fourfold increase compared to cisgender women.51 However, this was not replicated in a different case-control study of age- and gender-matched transgender men.50
Taken together, these findings demonstrate that the transgender populations that receive gender-affirming care may have different rates of CV risk and/or CVD compared to their cisgender counterparts.
Research Essentials for Investigators
There is often uncertainty on whether cardiovascular research studies are reporting sex or gender. This distinction is increasingly important with the growing transgender, nonbinary, and intersex populations in the healthcare sector. It is clear that treating sex or gender as a simple binary outcome is not sufficient in scientific research and can have adverse outcomes for SGM individuals. The Sex and Gender Equity in Research (SAGER) guidelines were developed to assist not only researchers, but also journal editorial teams in reporting sex and gender in cardiovascular research and publications.1,9
Every person has a sexual orientation, defined as a romantic or sexual attraction to other people independent of their gender identity; therefore, reliable measurements of these concepts are core to understanding population characteristics and outcomes in the same way as race, ethnicity, and other demographics. Without these data, healthcare disparities, discrimination, and mistreatment among SGM adults are difficult to assess.52 Additionally, lack of data makes it impossible to identify poor access to health, therapeutic advances, and cardiovascular outcomes for SGM adults. Routine assessment of sexual orientation and gender identity data sets would allow improved awareness of intersectionality (e.g. SGM people of color) in cardiovascular research.
Collection of sexual orientation and gender identity data requires a team that fosters trust, respect, and cultural competence, which can only be achieved through a team of healthcare professionals that are empathic, are able to understand the lifelong effects of marginalization, recognize and reduce their personal biases, use appropriate pronouns, and avoid invasive questions about identity when they are not relevant to research or cardiovascular care.9,52
Sexual Orientation Measures
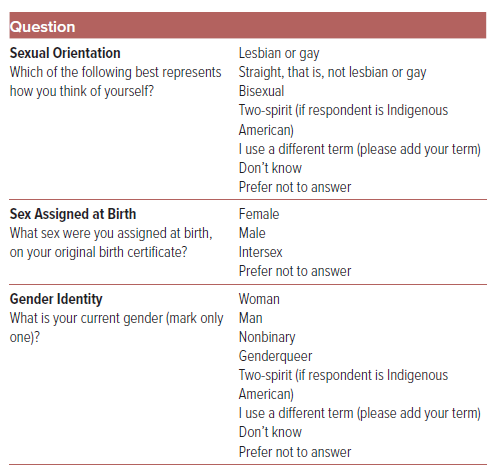
Inclusive demography should now encompass accurate assessment of an individual’s SOGIE data. Authors should report how sex and gender were considered in the design of the study to ensure adequate representation of groups.9 The SAGER guidelines provide measures for data collection that increase precision, inclusiveness, and privacy. Measures of sexual identity include three distinct approaches: asking whether someone identifies as a sexual minority; asking a respondent to select from a set of options that contain a combination of sexual orientation terminology; and asking a respondent to select from a set of sexual orientation terms (Table 1).1 It is important to recognize that terms used to affirmatively describe the SGM population have evolved and now include terms such as two-spirit, same-gender-loving, and queer. It is important to include a measure that is easy to implement, but also includes a ‘write-in’ category as a strategy to account for the ever-expansive list of sexual orientation categories.
Gender Identity Measures
Like sex, questions about gender identity can also be designed with categorical responses. These questions can be designed to ask respondents whether they identify as man, woman, neither, or another gender. The two-step measurement of gender consists of a two-question sequence that asks sex assigned at birth and current gender (Table 1).1 This two-step measure allows for the identification of cisgender men and women, transgender men and women, and nonbinary and genderqueer people.53 With regard to research design, this provides an accurate assessment of gender identity in contrast to prior measurements that only ask, “are you male or female?”, as this question conflates sex and gender. It also recognizes that a person’s gender identity can either be the same or different from the sex that they were assigned at birth. Lastly, it offers a range of gender identities rather than the historical binary categories associated with heteronormative research.54 In terms of feasibility, randomized multisite trials that added sexual orientation and gender identity questions to patient intake forms found that middle-aged and older adults endorsed the importance of collection gender identity data and also provided positive rather than negative feedback from those who completed a two-step measurement.55
Special Considerations
The SAGER guidelines recognize that the category of intersex is not currently available as a designation at the time of birth in the US. Standard practice in the US for children with intersex variations is to assign the child male or female shortly after birth.56 Designations such as ‘X’ and other nonbinary sex markers have not been widely adopted in cardiovascular research.57 Furthermore, in the absence of research on specific language to use in the measurement of intersex status especially for a population that has experienced traumatic medical treatment, the guidelines recommend using a question that directly asks the respondent if they have been diagnosed with an intersex condition.
Applications of the Guidelines to Cardiovascular Research
Today, authors should have a full grasp of the difference between sex and gender in all fields of research. Authors should report how sex and gender were considered in the design of the study, equally represent males and females, and justify exclusion of males, females, and sexual gender minorities. Next, results should be reported by sex. Analyses of sex and gender similarities and differences should be described regardless of positive or negative outcome. Importantly, the influence of sex and gender should be included in the causation, treatment effectiveness, and outcome of CVD. If sex- and gender-based analyses are not performed, authors should indicate reasons for lack of analysis in the limitations and discuss whether these demographic factors could have affected the results. Finally, the implications of sex and gender should be expanded and, if possible, it should be determined if the results can be generalized to all sexes and genders.
The SAGER guidelines provide researchers and editors with a framework to standardize sex and gender reporting. This tool is flexible enough for use in cardiovascular research to potentially improve the outcomes of SGM people. Integration of sex and gender into cardiovascular research provides more rigorous and ethical science through the incorporation of underserved populations. At a minimum, scientific journals should request that authors present data separately by sex and gender and explain these differences adequately. The routine collection of sexual orientation and gender identity in cardiovascular research will not only improve our identification of SGM populations, but also reduce glaring gaps in knowledge due to the lack of reliable data.
Conclusion
With the concerning rise in legislation limiting fundamental civil rights of SGM individuals in the past several years, it is paramount that healthcare practitioners provide a safe and inclusive environment for all patients. To achieve this, both clinicians and researchers should not only understand appropriate terminology, but also incorporate tools into their practice to promote health equity and inclusion. This is particularly important in cardiovascular research because a more comprehensive approach to data collection will better define CVD and risk factors in SGM individuals. Researchers should have a full grasp of the difference between sex and gender and incorporate appropriate data and terminology in all studies. Importantly, the influence of sex and gender should be included in the causation, treatment effectiveness, and outcome of CVD. In so doing, we can better promote health equity and outcomes in these vulnerable and marginalized populations.