Transcatheter aortic valve replacement (TAVR) is the standard of care for patients with symptomatic severe aortic stenosis at high or prohibitive surgical risk.1–4 The 2020 valvular heart disease guidelines from the American College of Cardiology and American Heart Association now include TAVR as a class I indication for patients aged 65–80 years and not high or prohibitive risk.5 The longer life expectancy of this patient population raises the issue of TAVR valve durability and the management of bioprosthetic valve failure of TAVR valves. In this review, we discuss bioprosthetic valve dysfunction and summarize existing data regarding redo-TAVR and surgery for failed TAVR. Finally, we propose an approach to evaluate patients with failed TAVR and plan for a second TAVR procedure as indicated.
Bioprosthetic Valve Dysfunction
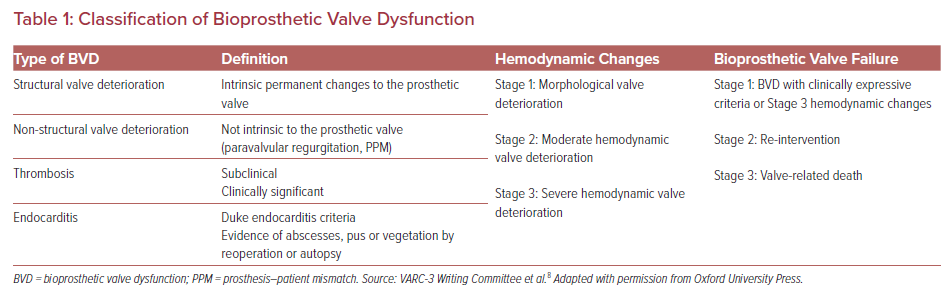
Dysfunction of a biological valve replacement is a well-known entity in the surgical literature that also occurs with TAVR valves. There have been multiple definitions of bioprosthetic valve dysfunction (BVD), the most recent being the Valve Academic Research Consortium (VARC-3) definition.6–8 The VARC-3 definition of bioprosthetic aortic valve dysfunction is comprised of categories including structural valve deterioration, non-structural valve deterioration, thrombosis, and endocarditis (Table 1).
BVD is also divided into three stages of deterioration on the basis of hemodynamic findings as follows:
- Stage 1, morphological valve deterioration.
- Stage 2, moderate hemodynamic deterioration, defined as an increase in mean transvalvular gradient ≥10 mmHg resulting in a mean gradient ≥20 mmHg, or new occurrence, or an increase of ≥1 grades of intraprosthetic aortic insufficiency (AI) resulting in greater than moderate AI.
- Stage 3, severe hemodynamic deterioration, defined as an increase in mean transvalvular gradient ≥20 mmHg resulting in a mean gradient ≥30 mmHg, or new occurrence, or an increase of ≥2 grades of AI resulting in severe AI.
These hemodynamic changes may be caused by structural valve deterioration, valve thrombosis or endocarditis. In addition to these definitions, VARC-3 also stresses the importance of clinical presentation and defines a subclinical presentation as BVD without symptoms or hemodynamic changes, with bioprosthetic valve failure categorized into three stages, ranging from the presence of clinical symptoms to reintervention to valve-related death.8
Management of Failed TAVR Valves
Redo-TAVR
Clinical data regarding redo-TAVR due to valve degeneration are scarce and reported outcomes are from case series or small multicenter studies. Table 2 provides an overview of study size, mechanism of TAVR failure, and clinical outcomes.
The first multicenter international collaborative work was reported by Barbanti et al. pooling data from 50 redo-TAVR procedures out of more than 13,000 procedures.9 Redo-TAVR was performed mostly for aortic regurgitation, including transvalvular or paravalvular regurgitation (78% of cases). The mean time from index to redo-TAVR was 812 days. Interestingly, this delay was significantly shorter among patients undergoing redo-TAVR for paravalvular leak (PVL) compared with valve stenosis or regurgitation. Redo-TAVR in these 50 cases was performed with no in-hospital death or disabling stroke. The mean aortic gradient and standard deviation improved after redo-TAVR, with values remaining slightly higher compared with those after the index TAVR (15.1 ± 6.7 versus 11.9 ± 7.7 mmHg, respectively). Follow-up mortality was 14.9% at a median time of 586 days.9
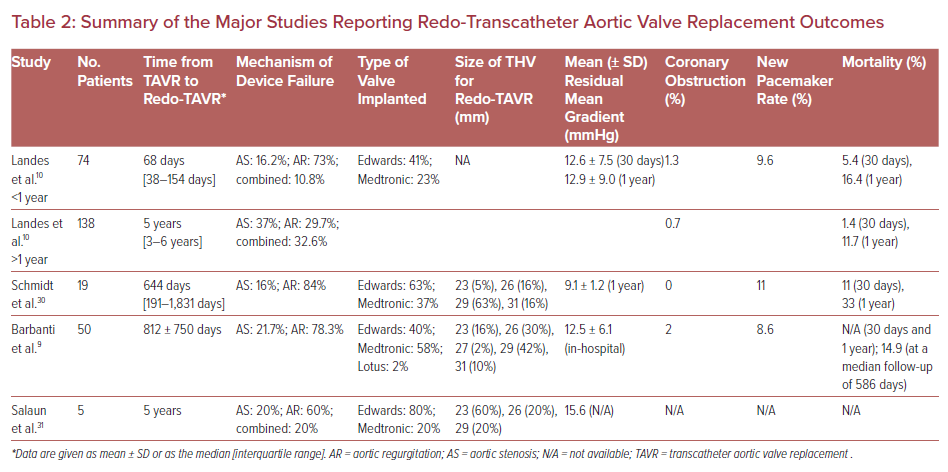
The largest cohort of redo-TAVR is a multicenter international registry comprising 37 centers through Europe, North America, and the Middle East.8 This registry identified 212 consecutive redo-TAVR procedures from a total of 63,872 index TAVR cases. The repeat procedures were divided into those within (n=74) and beyond (n=138) 1 year of the index TAVR. The indication for redo-TAVR was predominantly aortic regurgitation or combined stenosis and regurgitation. There was an interesting variation in pathology according to the timing of reintervention. Patients with early reintervention were more likely to have had procedural failure, whereas those with repeat intervention after 1 year were more likely to have transcatheter heart valve (THV) failure. The mean time between the index TAVR and redo-TAVR was 68 days and 5 years for procedural and THV failure, respectively. Procedural success of redo-TAVR was achieved in 85.1% of cases, with failures occurring in patients with high residual gradients after the intervention (14.1%) or residual regurgitation (8.9%).8 An important limitation of that study was the absence of differentiation between valvular regurgitation and paravalvular regurgitation or PVL among those with predominant aortic regurgitation as the etiology for failed TAVR.
Interestingly, in another study, there was a numerical trend towards a higher 30-day mortality among patients who underwent redo-TAVR for procedural versus THV failure, but this was not statistically significant (5.4% versus 1.4%, respectively; p=0.427).8 Periprocedural complications were low, and the 30-day survival was 94.6% in those undergoing redo-TAVR for procedural failure and 98.5% in those with THV failure (p=0.101). At 1 year, survival was 83.6% and 88.3% for patients with early and late valve dysfunction, respectively.8 The interpretation and generalizability of these outcomes may be limited given the highly selected population and unadjudicated outcomes.
Surgical Aortic Valve Replacement After TAVR
Surgical intervention for failed TAVR has been performed, but the results of larger registries are sobering. Data from the Society of Thoracic Surgeons database from 2011 to 2015 was used to evaluate the results of surgical intervention after TAVR for valve failure.10 In all, 123 patients were identified, with a median age of 77 years. Indications for reoperation included early TAVR device failures, such as PVL (15%), structural prosthetic deterioration (11%), failed repair (11%), sizing or position issues (11%), and prosthetic valve endocarditis (10%). The overall operative mortality in this group was 17.1% and the observed versus expected mortality ratios were higher for each group of preoperative risk category.11 This was a small, select cohort of initially high-risk patients who initially underwent TAVR, so these results may not be generalizable to a larger population, but they are concerning.
Considerations for Redo-TAVR
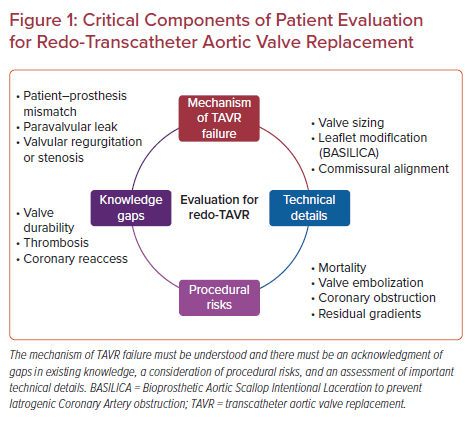
Given the risks of reoperation for these patients, evaluation for redo-TAVR and the associated risks is important to choose the appropriate therapy. When making a decision for a second TAVR intervention, it is important to understand some key details of the index procedure, namely post-procedure gradients and the presence of patient–prosthesis mismatch (PPM), PVL, and the mechanism of valve failure (see Figure 1).
Post-procedural Valve Gradient After the Index TAVR
Normal gradients following TAVR in native aortic valve stenosis average 5–15 mmHg. According to the revised VARC-3 criteria, device success is defined as a post-procedural mean gradient of <20 mmHg.8 In the presence of immediate or acute post-procedural mean gradient elevation, distinction between normal and abnormally low effective orifice area, or PPM, will direct further management. PPM may be a challenging echocardiographic diagnosis.
In a study by Flameng et al., PPM was found to be a predictor of structural valve degeneration (SVD) following surgical aortic valve replacement at a median time of 6 years, with patients mostly presenting with valve stenosis.11 PPM is defined as hemodynamically moderately and severely significant if the indexed effective orifice areas are 0.65–0.85 and <0.65 cm2/m2, respectively.12 Moderate and severe PPM following TAVR were reported in 25% and 12% of cases, respectively, in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) registry of over 62,000 patients.13 The impact of PPM on mortality remains a matter of debate and needs further investigation. Although mortality was higher among patients with severe PPM compared with non-severe PPM in the TVT registry at 1 year (17.2% versus 15.8%, respectively; p=0.011),13 Liao et al. described similar mortality at 2 years in their meta-analysis of 4,691 TAVR procedures.14 The size of the initial valve implanted is key information in the management of a failed TAVR with PPM. Indeed, as expected, in the TVT registry, the size of the prosthesis was inversely correlated with the severity of PPM (40%, 32%, and 24% of patients with severe, moderate, or no PPM, respectively had a 23 mm prosthesis implanted; p<0.0001).13 Therefore, in patients with confirmed PPM and valve failure, redo-TAVR is unlikely to be the best option and surgical intervention should be considered.
Presence of Paravalvular Leak
PVL is an important challenge in the current TAVR era and is associated with increased mortality.15 This has been decreasing with improved TAVR technology, but the high rates of aortic regurgitation in failed TAVR cases remain an important concern. Technical advancements (i.e. the use of device-sealing skirts, better preprocedural anatomical assessment, optimal oversizing) along with increased operator experience have permitted progressive reductions in PVL rates with time. However, in contrast with surgical aortic valve replacement, abolition of PVL following TAVR may be limited by the persistence of the diseased native valve apparatus and calcifications interfering with valve deployment. Echocardiography, specifically transesophageal echocardiography, is the imaging modality of choice to identify and differentiate intravalvular leak from PVL.
The persistence of significant PVL is to be expected following redo-TAVR because a second TAVR is unlikely to remedy the previous problem of stent frame malapposition against a calcified annulus. PVL closure may be effective, although no device is currently approved by the Food and Drug Administration. The most commonly used devices include the Amplatzer Vascular Plug (AVP) family (Abbott Structural). Overall, for both prosthesis undersizing and underexpansion resistant to balloon post-dilation, redo-TAVR will mostly fail to reduce pre-existing PVL.16
PVL due to an inadequate initial position of the TAVR valve, such as implantation too high or too low, may be addressed by a second procedure with an appropriately positioned valve.
Mechanism of Prosthesis Deterioration and Impact on Future Treatment
Characterization of the mechanism of TAVR failure plays a major role when considering redo-TAVR. Although redo-TAVR may be of interest for intravalvular regurgitation either due to SVD or non-SVD, its success for PVL seems less obvious, as discussed previously. In the largest case series to date, Landes et al. did not differentiate between intravalvular or paravalvular regurgitation among patients with aortic regurgitation, but did note that early valve failure was more frequently due to regurgitation.17 The overall modes of TAVR valve failure were stenosis (29.7%), stenosis–regurgitation (25.4%), and regurgitation (44.8%). These values are similar to previously published data on the mode of surgical valve failure in the TAVR VIVID (Valve-in-Valve International Data) registry.18
In those patients with TAVR valve stenosis as the primary mechanism of failure, it is possible for the stent frame to expand further to permit redo-TAVR. However, there is often a residual gradient, as is seen in the literature with valve-in-valve procedures for failed surgical valves.19 Registry data indicate that in those redo-TAVR patients with primarily stenosis, there was a significantly higher gradient at follow-up than in patients with failed TAVR due to combined stenosis–regurgitation or pure regurgitation (16.9 versus 10.4 mmHg, respectively, at 1 year). In addition, smaller prostheses (≤23 mm) had a significantly higher mean gradient than larger prostheses (>23 mm).13 Therefore, initial TAVR size is an important characteristic to include in the decision algorithm for redo-TAVR, particularly for stenotic failed TAVR.
Among reversible factors leading to TAVR failure, valve thrombosis remains an important issue for valve durability. The true incidence of TAVR valve thrombosis is unknown because, in most cases, it remains subclinical. In the OCEAN-TAVI registry including 485 patients, late leaflet thrombosis occurred in 16.9% of patients who had a follow-up CT up to 3 years.20 Although a direct causative link between leaflet thrombosis and valve durability has yet to be established, anticoagulation therapy has been shown to both reverse thrombotic leaflet reduced motion and decrease the transvalvular gradient.21
Patient Evaluation Prior to Redo-TAVR
The mainstays of patient evaluation prior to redo-TAVR include echocardiography, coronary angiography, and cardiac CT for procedural risk assessment and planning.
Coronary Angiography
Atherosclerotic coronary artery disease (CAD) and aortic stenosis share several risk factors, and up to 70% of patients have concomitant CAD at the time of index TAVR.22 Due to the heterogeneity of inclusion criteria and patient risk profile in studies, the impact of CAD revascularization before index TAVR on clinical outcomes remains unclear, with contradictory results in the literature.23,24 In the setting of redo-TAVR, coronary angiography is important to evaluate disease progression and to assess for risks of coronary occlusion. This can also be evaluated using CT.
Cardiac CT
Preprocedural cardiac CT is crucial prior to the index TAVR procedure. It allows precise characterization of aortic root anatomy, evaluation of coronary height, and annular sizing, as well as peripheral vascular assessment. For redo-TAVR planning, cardiac CT plays an important role for the evaluation of possible coronary obstruction and valve sizing.
Assessment of the risk of coronary obstruction following redo-TAVR requires analysis of the valve sinuses (height and width), valve to coronary distance (VTC), and the position of the commissures in relation to the coronary ostia. Coronary occlusion after redo-TAVR is rare and is mostly the consequence of a further shift of the native calcified leaflets against the coronary ostium. This risk of coronary occlusion is increased by a significant valve oversizing and small sinuses of Valsalva. As opposed to TAVR in failed surgical bioprosthesis, the initial TAVR prosthesis leaflets are less likely to directly occlude coronary ostia because the stent frame acts as a barrier.
Obstruction to coronary blood flow may occur due to sinus of Valsalva sequestration. Evaluation of this requires an understanding of the height of the sinotubular junction and its relationship to the commissural level of the first TAVR valve and the width of the sinuses of Valsalva. There may be a significant risk of sinus of Valsalva sequestration in the setting of a high commissural level of the TAVR valve (at the sinotubular junction or above) along with a low sinotubular junction. In such case, deployment of the new prosthesis may tilt the leaflets of the first prosthesis up against the stent frame, forming a hermetic cylinder at the sinotubular junction. When the first THV commissural level remains below the sinotubular junction, the risk is minimal. A smaller sinus of Valsalva diameter also increases the risk of sinus of Valsalva sequestration. In the study of Ochiai et al., the risk of sinus of Valsalva sequestration in case of redo-TAVR was assessed using post-index TAVR cardiac CT. Redo-TAVR was considered at increased risk for coronary obstruction in the presence of a first THV commissure level above the sinotubular junction and a distance between the THV and sinotubular junction <2 mm. Not surprisingly, patients with supravalvular valve design (Evolut R/Pro) had considerably higher CT-identified risks of sinus of Valsalva sequestration that those with an intra-annular valve design (SAPIEN 3; 45.5% versus 2%, respectively; p<0.001).25
VTC is a measure routinely used to plan valve-in-valve procedures in degenerated surgical bioprostheses. Patients with a virtual valve to coronary ostial distance (i.e. VTC) <4 mm had a higher risk of coronary obstruction.26 In the case of increased risk of sinus of Valsalva sequestration due to the first THV commissural level being located at the sinotubular junction level or above, or an increased risk of coronary obstruction with small VTC, additional strategies may have to be considered to modify the leaflets of the TAVR prosthesis (index valve). Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) is a leaflet modification technique developed to lacerate one or several leaflets of the first THV using an electrified guidewire that is punctured and snared through the leaflet before redo-TAVR.27 Although promising, all patients at risk of sinus of Valsalva sequestration or coronary occlusions are not candidates for BASILICA. Indeed, in the case of valve commissure and coronary ostia superposition, commissural posts will remain in front of the coronary ostia despite leaflet laceration. Commissural alignment at the first TAVR is of particular interest when treating younger patients who may become future candidates for redo-TAVR with potential BASILICA. As yet, with respect to the limited data, only the Evolut R/Pro platform seems to allow consistent commissural alignment with coronary ostia.28 Finally, when there is a risk of coronary obstruction after redo-TAVR and BASILICA is not an option, pre-emptive insertion of a guidewire and an undeployed stent in the left main coronary artery before THV deployment may be considered. If coronary obstruction occurs after prosthesis deployment, the stent is pulled back and implanted with protrusion in the aorta (chimney technique), although recannulation of these stents can be near impossible at a later date.29
Unanswered Questions
The prevalence of TAVR for the treatment of native aortic stenosis is rapidly increasing and permeating lower-risk populations. In the absence of long-term durability data, current experience suggests that TAVR valve failure will occur and a treatment strategy will be required. At present, redo-TAVR is emerging as a treatment option for failed TAVR valves, but concerns remain. Early experience suggests that cannulation of the coronary arteries following redo-TAVR can be unfeasible in up to 30% of patients depending on the type of index TAVR valve. The risks appear higher in those with a supra-annular THV compared with annular valves.30 Valve leaflet thrombosis is also a recognized entity despite being mainly subclinical. The impact of a second TAVR valve on future thrombosis is unclear, and whether antiplatelet, anticoagulation, or a combined therapy is the most effective regimen remains unknown. Finally, the development of new-generation THV with improved capabilities of commissural alignment and successive coronary reaccess will definitely facilitate redo-TAVR planning. When treating younger patients with a long life expectancy, the lifetime management of these patients must be considered and anticipated by the heart team while planning the index TAVR procedure.
Conclusion
Redo-TAVR is an emerging procedure for failed TAVR, with an encouraging safety profile and short-term clinical outcomes. The key to success will be an understanding of the index TAVR procedure and results, the mechanism of valve failure, and meticulous planning to minimize patient risk. The optimal treatment strategy for failed TAVR valves is still unknown, and longer-term data will be needed to best understand in whom redo-TAVR will be indicated.