Aortic valve disease is common in industrialized countries and its prevalence is expected to mirror the increase in life expectancy.1 Degenerative aortic stenosis is the most common valvular pathology requiring intervention.1,2 Choosing a prosthetic valve for patients meeting the criteria for aortic valve replacement requires careful consideration of valve durability, patient age, and contraindications to anticoagulation, and a shared decision-making approach.3
The past decade has witnessed the advent of transcatheter aortic valve replacement (TAVR), which has revolutionized the management of aortic stenosis. TAVR is now recommended in symptomatic patients >80 years old with a life expectancy of 1–10 years, symptomatic patients aged 65–80 years with no contraindication to TAVR, and symptomatic patients of any age with prohibitive surgical risk with a life expectancy of more than 12 months.3
Surgical aortic valve replacement (SAVR) is recommended in the younger patient population, patients at high risk for reinterventions, and those needing other cardiac surgeries.3 Currently, there are no approved transcatheter options for the management of pure aortic regurgitation.
Irrespective of the prosthetic aortic valve chosen, both SAVR and TAVR prosthesis are prone to prosthetic valve dysfunction, including endocarditis, thrombosis, bioprosthetic degeneration, and paravalvular leak.4
With advancements in cardiac imaging, especially multidetector cardiac CT (MDCT), clinical and subclinical prosthetic valve thrombosis is being more readily diagnosed. The aim of this review is to provide a comprehensive approach to the evaluation of patients with suspected or confirmed prosthetic aortic valve thrombosis.
Prosthetic Valve Thrombosis
Definition
Prosthetic valve thrombosis is defined as thrombus formation on a prosthetic valve that is initiated by either incomplete endothelialization or endothelial disruption secondary to exacerbating factors, such as endocarditis, degenerative changes, or pannus formation.2
The use of MDCT has facilitated the identification of two new entities of hypoattenuated leaflet thickening (HALT) and reduced leaflet motion (RLM), both of which are now considered to be in the spectrum of prosthetic valve thrombosis. Prosthetic valve thrombosis may or may not be associated with thromboembolic events and/or valvular dysfunction.2
The Valve Academic Research Consortium 3 (VARC3) categorized stages of bioprosthetic valve thrombosis as morphological deterioration without significant hemodynamic changes (stage 1), moderate hemodynamic deterioration (stage 2), and severe hemodynamic deterioration (stage 3).5
Subclinical valvular thrombosis is defined as morphological findings suggestive of valvular thrombosis with no/mild hemodynamic significance and an absence of symptoms or evidence of thromboembolic events.5
Clinically significant valvular thrombosis is defined as:
- Thromboembolic events or worsening valvular deterioration with stage 2 or 3 changes and confirmatory imaging findings of HALT on MDCT or transesophageal echocardiography (TEE) findings;5 or
- Stage 3 valvular deterioration and confirmatory imaging findings of HALT on MDCT or TEE findings.5
Prevalence
Prosthetic valve thrombosis is more common in right-sided implants and mitral valve prostheses than in prosthetic aortic valves.6 However, the incidence and prevalence of aortic valve thrombosis are likely to be underestimated because routine follow-up imaging with MDCT is not performed in the absence of symptoms or hemodynamic changes noted by echocardiography.
Patient and hemodynamic factors that increase the risk of prosthetic aortic valve thrombosis include reduced left ventricular systolic function, AF, prior history of valvular thrombosis, and older generation implants.3
SAVR
While the overall prevalence of mechanical prosthetic valve thrombosis is reported to be as high as 5.7%, the prevalence of mechanical aortic valve prosthesis is estimated to be close to 1%.2
Studies of surgical bioprosthetic valve thrombosis report a 0.61–0.7% prevalence of valve thrombosis, with the majority of the cases being identified within 6 months of implantation.2,3
TAVR
TAVR valves have been reported to be more prone to thrombosis than surgically implanted bioprosthesis.2 Incidence of TAVR thrombosis is also believed to be higher than previously reported, based on follow-up data from MDCT and 4D CT, and the inclusion of HALT and RLM in the spectrum of prosthetic thrombosis.
Most recently, sub-studies of PARTNER 3 and Evolut Low Risk investigated the prevalence and progression of HALT and RLM in low-risk patients undergoing TAVR and SAVR. A PARTNER 3 sub-study showed a higher prevalence of HALT at 30 days in TAVR versus SAVR valves (13% versus 5%; p=0.03) but not at 1 year (28% versus 20%; p=0.19). On the other hand, the Evolut Low Risk sub-study showed a higher prevalence of HALT in both TAVR and SAVR valves with no significant difference at 30 days and 1-year follow-up. Both studies confirmed the dynamic nature of HALT, with spontaneous resolution and progression with an overall increase in prevalence at 1 year.7,8
The three largest observational studies reported a rate of 7–12% for clinical and subclinical valvular thrombosis in patients after TAVR.9,10 Risk factors for prosthetic valve thrombosis included larger valve sizes and balloon-expandable valves. Recently, a meta-analysis investigating the results of 20 studies with more than 12,000 patients showed that the prevalence of subclinical leaflet thrombosis and clinical valvular thrombosis in TAVR valves were 15.1% and 1.2%, respectively.11
Pathophysiology
Prosthetic valve thrombus formation entails a complex interplay between the prosthetic valve surface in addition to hemostatic and hemodynamic factors.
Surface factors include the process of endothelialization and prosthesis positioning. Prosthetic materials are thrombogenic in nature; this triggers the deposition of a fibrin layer, which is then replaced by neointimal tissue approximately 3 months after implantation. This is then followed by tissue fibrosis. Any disruption in this process could promote thrombus formation.
Hemostatic factors include high systemic inflammatory states, such as chronic kidney disease, obesity, and smoking.
Local factors include excessive surgical tissue manipulation or pre-/post-dilatation, which increases tissue plasminogen expression.
Hemodynamic factors such as heart failure and abnormal prosthesis hemodynamics increase hypercoagulability of the local blood pool by causing excessive turbulent flow, worsening stasis, and delaying endothelialization.2
Therefore, all prosthetic valves require short- and long-term treatment with either vitamin K antagonists (VKAs) and/or antiplatelet agents to promote uninterrupted endothelialization of the prosthetic material.6
Diagnosis
Clinical Presentation
Patients with prosthetic valves should be followed on a regular basis and counseled on symptoms suggestive of prosthetic valve dysfunction.
Acutely ill patients with prosthetic valves should undergo detailed assessment of prosthetic valve function, including a consideration of thrombosis as the potential etiology of their decompensation.12 Symptoms of prosthetic valve thrombosis include heart failure symptoms related to prosthetic valve stenosis or regurgitation, and symptoms suggestive of thromboembolic events (such as transient ischemic attack/stroke, unexplained abdominal pains, or ischemic limbs).
A thorough review of the anticoagulation/antiplatelet regimen and the duration of treatment should be performed and emphasized to the patient with each visit.
Excluding infective endocarditis and AF as inciting factors for prosthetic valve thrombosis is also essential.2,3,6
Mechanical valve thrombosis has a relatively acute/subacute presentation while bioprosthetic valves are less prone to acute thrombosis, especially after the initial endothelialization phase.
Bioprosthetic and TAVR valve thrombosis is usually suspected based on a change in prosthetic valve hemodynamics as noted by echocardiography, which triggers further investigation.
Imaging
Echocardiography
A comprehensive transthoracic echocardiogram (TTE) study is the first-line imaging modality recommended for the assessment of prosthetic valve function. A complete 2D assessment of left ventricular size and systolic function, the left ventricular outflow tract (LVOT), and prosthetic disc/leaflet thickness and mobility should be performed.
Differentiating a thrombus from vegetations, degenerative changes, masses, or sutures can prove to be challenging by echocardiography and requires the use of multimodality imaging.12 Doppler interrogation of the valve establishes prosthetic valve hemodynamics and should be performed at baseline to enable comparative evaluation. Any new transvalvular regurgitation necessitates further investigation for prosthetic valve obstruction.
Echocardiographic parameters suggestive of prosthetic valve obstruction include a peak velocity of >3 m/s, a mean gradient of >20 mmHg, a dimensionless velocity index (DVI) of <0.3, an effective orifice area (EOA) of <1.2 cm, acceleration time (AT) of >80 ms and a rounded Doppler jet contour (Table 1).2,12 In comparative assessment, a mean transvalvular gradient increase of more than 50% from baseline or >10 mmHg is considered pathological in the absence of high output states to account for such an increase.3
Evaluation of prosthetic aortic valves by TEE can provide additional information on leaflet thickening, leaflet mobility, and the presence of vegetations or thrombi, in addition to the presence of transvalvular or paravalvular regurgitation.12–14 However, visualization of the valve can be adversely affected by acoustic shadowing especially in the setting of mechanical prosthetic aortic valves.
Although TEE has been proven to be a great tool in assessing the mitral valve hemodynamics, it has limitations in the assessment of aortic valve hemodynamics owing to the anterior position of the valve and limited views of the aortic valve and LVOT from deep transgastric views.
Despite its limitations, TEE can provide invaluable information in differentiating pannus from thrombotic lesions. Visualized pannus on TEE tends to be small and ultrasound dense compared to the large, soft ultrasound density lesions seen with thrombosis formation. Unfortunately, hemodynamic findings provide little value in differentiating pannus from thrombotic lesions.12
Patients with prosthetic valves should undergo an initial detailed assessment of the prosthetic valve to establish baseline hemodynamics (class I recommendation). Subsequent imaging follow-up with TTE is recommended to be performed annually in patients with transcatheter aortic valves (class IIa recommendation), and at 5 and 10 years and then annually in patients with bioprosthetic surgical valves (class IIa recommendation). Any change in clinical status of the patients should initiate prompt assessment with a TTE and, if required, further investigation with TEE and MDCT (class I recommendation).3
In patients with non-conclusive findings, stress echocardiography can be performed to assess pressure change across the prosthetic valve and reproduction of the symptoms. Overall, a large increase in pressure gradient across the prosthesis is highly suggestive of prosthetic valve obstruction.12
Cine Fluoroscopy
The main use of cine fluoroscopy is in the evaluation of mechanical disc mobility. Impaired excursion of the mechanical valve discs suggests prosthetic valve obstruction. In cases with a high suspicion of valvular thrombosis, a more detailed assessment with contrast dye may be warranted.3,15
CT
Routine assessment of prosthetic valves by MDCT is not usually performed in the absence of suspicion of prosthetic valve dysfunction. Advances in CT acquisition techniques and processing analysis have enabled accurate assessment of prosthetic valve function.
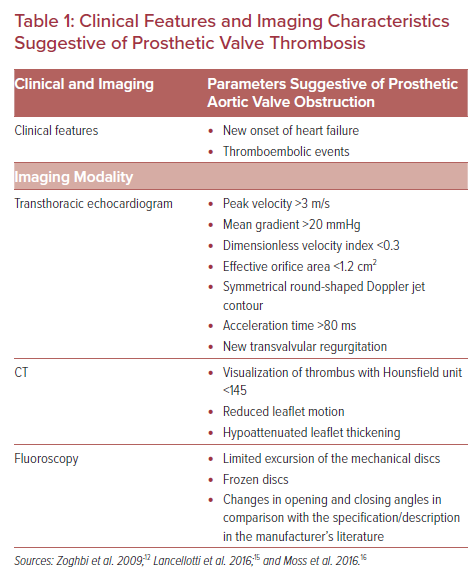
MDCT facilitates the evaluation of a mechanical valve’s opening and closing angles, a bioprosthetic/transcatheter valve’s dynamic leaflet mobility and thickness, in addition to an in-depth analysis of surrounding tissue characterization (Figure 1).16 The majority of valvular thrombotic lesions detected by MDCT have a Hounsfield unit (HU) of <90 (87 ± 59). On the contrary, pannus tends to have a HU >145 (322 ± 122), with lesions with a HU of 90–145 being indeterminate.16,17
In addition, the location and timing of occurrence of a lesion provide further details that aid in determining its etiology. Pannus is predominantly circumferential compared to more irregularly shaped thrombotic lesions. Thrombotic lesions can occur at any time, but most frequently occur in the first year after implantation and usually arise on both the aortic and LVOT sides of the valve. However, pannus tends to form over a longer period of time (>1 year) and mostly occurs on the LVOT side of the prosthetic valve (Figure 1).16
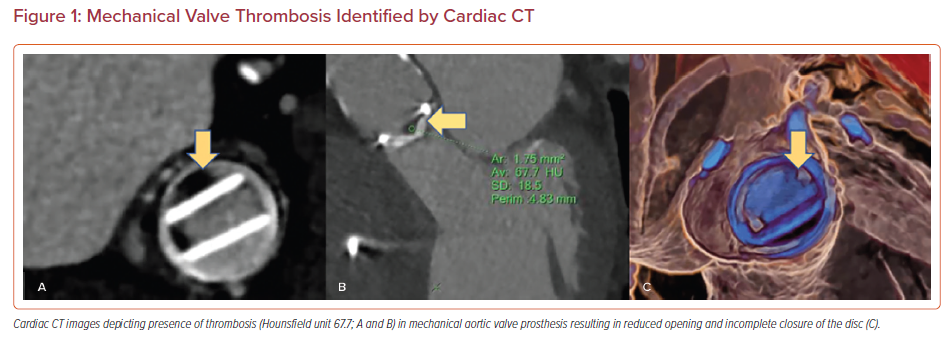
Based on MDCT data, two new entities in the spectrum of prosthetic valve thrombosis have been described for surgical bioprosthetic and transcatheter aortic valves, namely HALT and RLM. Both HALT and RLM are diagnosed using 4D, volume-rendered imaging protocols and can present as subclinical or clinically significant valvular thrombosis.5
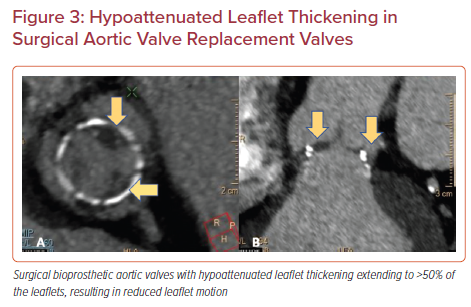
HALT is defined as an increase in the thickness of the prosthetic valve leaflets. The increase in thickness results in a meniscal shape that can be visualized on long axis images extending from the base of the leaflet to the tip. It is graded according to the percentage of leaflet involved. HALT should be reported based on its location, extent of involvement and leaflet thickness using a four-tier grading system: ≤25%; >25% and ≤50%; >50% and ≤75%; and >75%.18,5
Reduced leaflet motion (RLM) is defined as being present or absent in the presence of HALT with a four-tier grading system: no reduction in leaflet excursion; <50% reduction in leaflet excursion; ≥50% reduction in leaflet excursion; or immobile leaflets.5 In the absence of HALT, RLM is rare and should be called with extreme caution to avoid unnecessary intervention or treatment (Figures 2 and 3).7,18–20
Management
Mechanical Prosthetic Aortic Valves
In patients with suspected mechanical valve thrombosis presenting with acute onset of symptoms suggestive of heart failure, shock, or thromboembolic events, urgent imaging is recommended as detailed above to assess the extent of valvular thrombosis and valvular dysfunction.3
In cases of confirmed mechanical prosthetic valve thrombosis, the decision for optimal therapy either with emergent surgery or slow infusion with fibrinolytic therapy is based on multiple factors (Table 2).
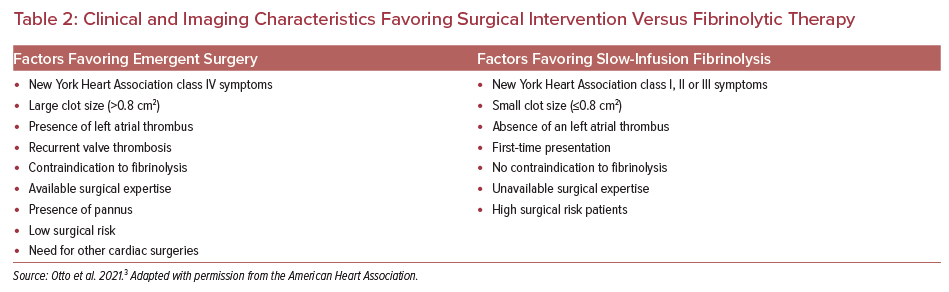
Slow-infusion of fibrinolytic therapy of 25 mg tissue-type plasminogen activator over 25 hours has a hemodynamic success rate of >90% with an embolic and major bleeding risk of <2% in patients meeting the selection criteria.3 Follow-up imaging is performed to reassess prosthetic valve gradients and success of thrombolytic therapy. Figure 4 demonstrates a case of mechanical valve thrombosis successfully treated with IV thrombolysis.
Surgical Bioprosthetic and Transcatheter Aortic Valves
In patients with suspected bioprosthetic valve thrombosis, detailed imaging is warranted for detailed assessment of prosthetic valve function and leaflet mobility. With confirmed or highly suspected bioprosthetic valve thrombosis, therapy with VKAs is recommended (class IIa recommendation).
Small studies have assessed the duration and timing of anticoagulation therapy.21–23 Overall, there is limited data on the duration of anticoagulation therapy for bioprosthetic valve thrombosis. However, follow-up imaging analysis has shown thrombosis has resolved within 14 days of therapy initiation, demonstrated by a reduction in the transvalvular gradient. Currently, there are no guidelines on the optimal duration of anticoagulation therapy.
Anticoagulation with VKAs is the recommended regimen over direct oral anticoagulants.3,24 Currently, there is limited data available for anticoagulation therapy in patients with HALT and RLM. The evidence for treatment of subclinical leaflet thrombosis is discussed later in this review.10
Prevention
Mechanical Prosthetic Aortic Valve
Prevention of mechanical aortic valve thrombosis is essential in patients with these valves. Hence, these patients require lifelong anticoagulation with a VKA with specific international normalised ratio (INR) target ranges.
An INR target of 2.5 (2–3) is recommended for patients with no other risk factors for thromboembolism.3 In patients with risk factors for thromboembolism including AF, a prior history of thromboembolism, hypercoagulable states, older-generation mechanical valves and left ventricle systolic dysfunction, an INR target of 3 (2.5–3.5) is recommended.3 Studies on the mechanical On-X valve (On-X Life Technologies) showed that these patients can be managed with an INR goal of 1.5–2 and low-dose aspirin therapy (class IIb recommendation).3
Should patients with a mechanical prosthetic valve require an interruption in anticoagulation therapy for non-cardiac procedures, it is important to note that those with no risk factors for thromboembolism do not require bridging.3
Addition of antiplatelet agents to VKAs increases the risk of the bleeding. However, in patients with a mechanical prosthetic aortic valve who need antiplatelet therapy, low-dose aspirin (75–100 mg) is recommended (class IIa recommendation).3
Bioprosthetic Surgical Valves
In the absence of risk factors for bleeding, it is recommended that patients with a surgically implanted bioprosthetic aortic valve receive VKAs therapy for at least 3 months and up to 6 months (class IIa recommendation).
The therapy with VKAs can be switched to direct oral anticoagulants after 6 months if anticoagulation therapies need to be continued (e.g. in AF). If there is no need for oral anticoagulation, lifelong therapy with low-dose aspirin is recommended for this patient cohort (class IIa recommendation).3
Transcatheter Aortic Valves
Management of patients after TAVR remains controversial, and multiple studies are being conducted to address the need for anticoagulation therapy in patients with transcatheter valves.
In the current guidelines for the management of valvular heart disease, single antiplatelet agents are preferred in patients with TAVR valves and no other indication for anticoagulation or dual antiplatelet therapy (class IIa recommendation).3 Currently, the use of dual antiplatelet regimens have a class IIb recommendation because of an increased risk of bleeding.
In patients with suspected TAVR thrombosis or subclinical leaflet thrombosis and patients requiring anticoagulation for other indications, selective VKA therapy is preferred over direct oral anticoagulants (class IIb recommendation).3 The GALILEO trial compared a regimen of low-dose rivaroxaban (10 mg daily) and aspirin to dual antiplatelet therapy with aspirin and clopidogrel. The study was terminated prematurely because of a significant increase in major adverse outcomes and safety concerns in the rivaroxaban and aspirin arm.24
Since the publication of the 2020 American College of Cardiology/American Heart Association guideline for the management of patients with valvular heart disease, results of other randomized controlled trials (RCTs) have been published assessing anticoagulation and antiplatelet therapies in patients after TAVR.
POPular-TAVI trial cohorts (NCT02247128) are the largest RCTs addressing the issues of single versus dual antiplatelet therapy and clopidogrel with or without anticoagulation in patients post TAVR.
POPular-TAVI Cohort A
This open-label RCT studied single antiplatelet therapy with aspirin versus dual antiplatelet regimen with aspirin and clopidogrel in patients with no other indication for anticoagulation.25
Primary outcome (all bleeding and non-procedure related bleeding) analysis showed that there was less bleeding in the aspirin-treated cohort than in the aspirin and clopidogrel cohort (15.1% versus 26.6%; 95% CI [0.42–0.77]; p=0.001), with a predominant increase in primary outcomes in the first 45 days of the procedure.
Aspirin was also superior to a combination of aspirin and clopidogrel in the secondary composite outcome 1 (bleeding, death from cardiovascular causes, non-procedure-related bleeding, stroke from any cause, or MI) with a risk ratio of 0.74 (95% CI [0.57–0.95]; p=0.04) and non-inferior in secondary composite outcome 2 (thromboembolic events, including death from cardiovascular causes, ischemic stroke, or MI), with an absolute difference of −0.2 (95% CI [−4.7–4.3]; p=0.004).25
POPular-TAVI Cohort B
This open-label RCT addressed the question of the addition of clopidogrel to anticoagulation regimens for patients undergoing TAVR and in need of anticoagulation for other indications.26 The primary outcome was overall and non-procedure-related bleeding. Data analysis showed that patients receiving clopidogrel in addition to anticoagulation had more overall bleeding episodes than those on anticoagulation without clopidogrel (34.6% versus 21.5%; 95% CI [0.43–0.90]; p=0.01), and had more non-procedure-related bleeding (21.7% versus 34.0%; 95% CI [0.44–0.92]; p=0.02).
Secondary composite outcomes 1 and 2 were defined as per the POPular TAVI cohort A arm. Secondary outcome analysis showed that oral anticoagulation without clopidogrel was superior and non-inferior to combination therapy for secondary composite outcomes 1 and 2, respectively.26
A recent meta-analysis of four major RCTs on the use of dual versus single antiplatelet therapy in patients undergoing TAVR showed no benefit in the prevention of all-cause mortality, stroke, or MI in patients receiving dual antiplatelet therapy over a single antiplatelet regimen. It was also shown that there was a significant reduction in any bleeding and life-threatening or major bleeding events in the single antiplatelet therapy cohort.25,27–30
Subclinical Leaflet Thrombosis in TAVR and SAVR Bioprosthetic Valves
The two largest observational studies assessing subclinical leaflet thrombosis are the RESOLVE and SAVORY registries.10 Collectively, 890 patients were studied in these two registries.
Subclinical leaflet thrombosis was identified in 12% of the patients with a predominant involvement of the TAVR as compared to SAVR valves (96% versus 4%; p=0.001). Clinical outcome analysis showed that subclinical leaflet thrombosis was associated with an increase in transient ischemic attacks (TIAs) and combined stroke/TIAs with HRs of 7.02 and 3.27, respectively, predominantly driven by TIA events.
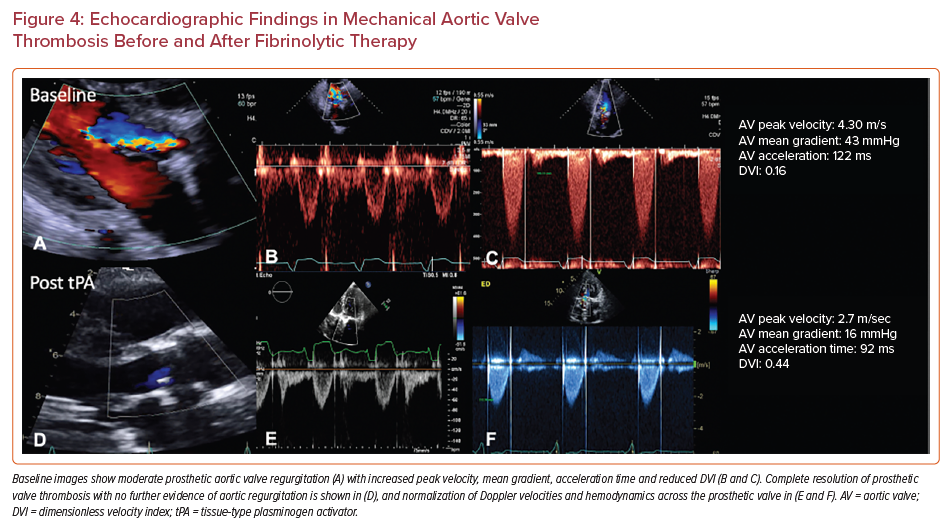
Patients on oral anticoagulation showed complete resolution of subclinical valvular thrombosis (Figure 5). However, these studies were not powered to address the risk associated with major adverse outcomes of anticoagulation therapies in the setting of subclinical leaflet thrombosis.10
Conclusion
Both surgical and transcatheter prosthetic aortic valves are prone to thrombosis. A change in clinical status or valve hemodynamics should raise suspicion for prosthetic valve dysfunction, including prosthetic valve obstruction. Multimodality imaging, especially with the use of MDCT, is key to establishing diagnosis.
The optimal management strategy depends on the type of valve in addition to various patient-related and imaging-specific factors. Postoperative anticoagulation or antiplatelet therapies should be emphasized in patients with prosthetic aortic valves to decrease the risk of prosthetic valve thrombosis.