Dual antiplatelet therapy (DAPT), comprising aspirin and a P2Y12 receptor inhibitor, is the cornerstone of treatment in patients who have undergone percutaneous coronary intervention (PCI).1,2 Clopidogrel was the first broadly used P2Y12 receptor inhibitor and conferred a significant reduction in ischemic events after PCI.3,4 However, clopidogrel’s metabolic activation is unpredictable, leading to variability in response among patients. Genetic polymorphisms, among other factors, have been implicated in ‘clopidogrel resistance’.5
Several studies have found an association between impaired response to clopidogrel (often termed high on-treatment platelet reactivity [HPR]) and adverse ischemic events.6–8 On the other hand, low platelet reactivity (LPR) has been recognized as a risk factor for bleeding events, which significantly influence the clinical outcome of patients with coronary artery disease (CAD) during and after myocardial revascularization.6,9
These observations, along with the advent of more potent P2Y12 inhibitors (prasugrel and ticagrelor), gave birth to the concept of antiplatelet treatment tailoring; this concerns the adaptation and individualization of antiplatelet therapy type and duration based on various factors, including clinical presentation, the patient’s clinical profile, ischemic/bleeding scores, and laboratory/point-of-care tests. More specifically, potential strategies involve upfront selection of a more potent P2Y12 inhibitor (such as prasugrel or ticagrelor) instead of clopidogrel based on genotyping, and escalation (switching from clopidogrel to prasugrel or ticagrelor) or de-escalation (switching from prasugrel/ticagrelor to clopidogrel) of the initial antiplatelet treatment according to platelet function testing (PFT) results.10
Moreover, various scores – including the Predicting Bleeding Complications In Patients Undergoing Stent Implantation and Subsequent Dual Anti Platelet Therapy (PRECISE-DAPT) score, the DAPT score, ARC-HBR trade-off risk model and PARIS – have been proposed for uncoupling bleeding and ischemic risks in patients undergoing PCI and, thus, guiding DAPT duration in this context.11–14
Patients with C-PCI constitute a special PCI subpopulation where antiplatelet treatment is challenging. Although no universal definition exists, C-PCI according to the European Society of Cardiology (ESC) involves at least one of the following procedural aspects: implantation of three or more stents; treatment of three or more lesions; bifurcation PCI with two stents; total stent length >60 mm; or chronic total occlusion (CTO) PCI.15 Additionally, left main (LM) or proximal left anterior descending (LAD) coronary artery PCI, saphenous vein graft PCI, bifurcation lesion with a side branch ≥2.5 mm, use of rotational atherectomy, lesion length ≥30 mm or the presence of thrombus in the coronary lesion have also been considered as procedural characteristics defining a coronary intervention as C-PCI.16
In addition to procedural complexity, patient complexity because of multiple comorbidities and high-risk clinical features (such as smoking, diabetes, chronic kidney disease, peripheral arterial disease, hypertension and/or poor left ventricular function) is increasing over time in patients presenting for PCI.17 C-PCI patients are at increased risk for ischemic events and this risk is greater as procedural complexity increases.18–19 It is noteworthy that C-PCI patients seem to be at a higher risk for major bleeding as well.20 DAPT of increased potency and duration might reduce adverse ischemic events but increase the risk of bleeding as well, which can compromise the clinical outcome.
The optimal antiplatelet treatment strategy in C-PCI remains a controversial and moving field. PFT and genotyping have been considered as potentially useful tools in individualizing antiplatelet treatment in attempts to balance ischemic and bleeding risks. The aim of this review is to summarize the existing evidence and critically appreciate the role of PFT and genotyping for antiplatelet treatment tailoring in C-PCI patients.
PFT and Genotyping in PCI
DAPT, comprising aspirin and a P2Y12 receptor inhibitor, has improved PCI clinical outcomes by reducing ischemic complications.3 The second-generation thienopyridine clopidogrel was the first broadly used P2Y12 receptor inhibitor, as it exhibited a better safety profile than the first-generation thienopyridine ticlopidine. However, platelet function measurements showed that the response to this regimen varied, with some patients exhibiting poor response, a condition often termed HPR or clopidogrel resistance, as mentioned above.21 Several factors have been implicated in the variation in response to clopidogrel, including diabetes, acute coronary syndrome, a high BMI, renal failure, older age, heart failure, inflammation, smoking, drug–drug interactions and genetic polymorphisms (mainly of CYP2C19).21–23
The results of multiple studies indicating a clear association between HPR and adverse ischemic events after PCI paved the way for research efforts investigating the role of treatment tailoring.6,21 This approach initially involved increasing clopidogrel dose but this failed to reduce the incidence of death from cardiovascular causes, nonfatal MI, or stent thrombosis in HPR patients after PCI, perhaps because of a modest pharmacodynamic effect.24
With the advent of the more potent P2Y12 inhibitors prasugrel and ticagrelor, escalation of antiplatelet treatment by switching clopidogrel to a novel agent became an additional option. On the other hand, an increased response to P2Y12 receptor inhibitor, leading to LPR, has been recognized as a risk factor for bleeding events, which compromise clinical outcomes after PCI.9 As a result, treatment de-escalation has also been proposed as a potentially beneficial strategy for patients at a high risk of bleeding.25
Several methods of PFT are available for the ex vivo measurement of platelet reactivity to adenosine diphosphate, including point-of-care assays (e.g. the VerifyNow P2Y12 system [Werfen], the Multiplate analyzer [Roche], and thromboelastography [TEG] platelet mapping) and laboratory-based techniques (e.g. light transmission aggregometry [LTA] and vasodilator-stimulated phosphoprotein [VASP]).
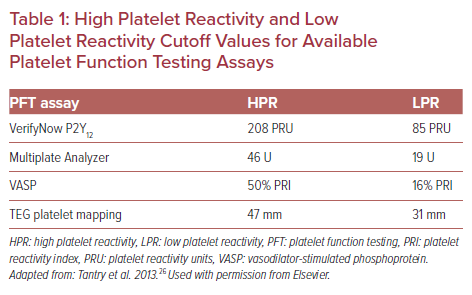
There is a consensus that point-of-care assays should be preferred, and specific cut-off points for HPR and LPR have been defined for each assay (Table 1).8,26 Platelet reactivity assessed by a PFT assay in a P2Y12-treated patient should ideally be within the therapeutic window between LPR and HPR, which is associated with the lowest risk of ischemic and bleeding events according to observational data.27 Major randomized studies testing PFT-guided escalation of antiplatelet treatment in PCI patients failed to show clinical benefit, which calls its role in clinical practice into question.24,28–30 However, a PFT-guided de-escalation approach was found to be non-inferior for the primary endpoint of net clinical benefit (cardiovascular death, MI, stroke or bleeding grade ≥2 according to Bleeding Academic Research Consortium [BARC] criteria) compared with potent platelet inhibition for 12 months after PCI for acute coronary syndrome (ACS) in the TROPICAL-ACS randomized study (7% in the guided de-escalation group versus 9% in the control group; p for non-inferiority=0.0004; HR 0.81; 95% CI [0.62–1.06]). It is noteworthy that there was no increase in the combined risk of cardiovascular death, MI, or stroke in the early de-escalation group.31
As already mentioned, HPR in clopidogrel-treated patients may be related to polymorphisms of genes that encode cytochromes responsible for clopidogrel’s metabolic activation. Research on genotyping has focused on polymorphisms of the CYP2C19 gene, the most common and relevant being CYP2C19*2 loss of function (LoF) polymorphism, causing CYP2C19 activity to be lost. The CYP2C19*3 allele is another LoF polymorphism, which has a low prevalence in white people.32 However, only 6–12% of the variability on clopidogrel response can be attributed to differences in genotype.5,33 It is reasonable to use genotyping only for clopidogrel-treated patients and point-of-care assays are recommended over laboratory-based methods.8
Some randomized studies have shown efficacy of CYP2C19 genotyping in antiplatelet treatment tailoring for both elective and ACS PCI patients.34–37 However, the recently announced TAILOR-PCI study reported – though marginally – a non-significant (4% versus 5.9%; HR:0.66; p=0.056) reduction in major adverse cardiovascular events (MACE) (non-fatal myocardial infarction or stroke, cardiovascular death, severe recurrent ischemia or stent thrombosis) at 1 year in the genotyping compared with the non-genotyping group.38
Beyond the CYP2C19*2 LoF polymorphism, clinical factors are believed to have contributing roles in HPR and thrombotic complications. In this context, the ABCD-GENE (Age, Body mass index, Chronic kidney disease, Diabetes mellitus, and Genotyping) score was developed, which incorporates four clinical (age >75 years, BMI >30 kg/m2, chronic kidney disease [estimated glomerular filtration rate <60 ml/min/1.73m2], and diabetes) and one genetic (CYP2C19*2 LoF alleles) independent predictors of HPR. The ABCD-GENE score has been shown to independently correlate with all-cause death, as well as with the composite of all-cause death, stroke, or MI, both as a continuous variable and by using a cut-off point of ≥10.39
Current guidelines on DAPT recommend against (class III) the routine use of PFT and genotyping as antiplatelet treatment modification guidance in the context of PCI.2,15 However, according to the recently published ESC guidelines on non-ST-segment elevation ACS: “De-escalation of P2Y12 receptor inhibitor treatment… may be considered as an alternative DAPT strategy, especially for ACS patients deemed unsuitable for potent platelet inhibition. De-escalation may be done unguided based on clinical judgment, or guided by platelet function testing, or CYP2C19 genotyping, depending on the patient’s risk profile and availability of respective assays.” (Class IIb, level of evidence A.)40 Moreover, experts agree that use of PFT/genotyping may be reasonable in specific high-risk clinical scenarios, including C-PCI.8
Rationale for Use of PFT and/or Genotyping in C-PCI
During the last decade, C-PCI procedures have been increasingly performed. Moreover, as mentioned before, a rise in the high-risk clinical features (smoking, diabetes, chronic kidney disease, peripheral arterial disease, hypertension, and poor left ventricular function) of treated patients has also been observed.41 C-PCI patients are at a higher risk of ischemic events, and this risk becomes greater as procedural complexity increases.18,19,42 DAPT of increased potency (with prasugrel or ticagrelor) might reduce adverse ischemic events.
On these grounds, ESC guidelines for myocardial revascularization offer the option of administering prasugrel or ticagrelor in specific high-risk situations of elective PCI; however, the level of evidence for this is weak (IIb, C).1 Moreover, prolonged DAPT has also been proposed as a potentially beneficial strategy for this high-risk group.19 Nevertheless, C-PCI patients seem to be at increased risk for major bleeding as well.20 Consequently, more potent and/or prolonged DAPT might compromise clinical outcome by increasing bleeding events.
As C-PCI procedures are performed more often and in sicker patients, optimization of DAPT strategies is imperative to optimize clinical outcomes. Here, antiplatelet treatment tailoring according to each patient’s ischemic and bleeding risk is a reasonable approach. PFT and genotyping may serve as tools to escalate or de-escalate DAPT to achieve the desired level of antiplatelet effect. Moreover, PFT could also be used to check compliance with treatment, which is crucial in this context.
Evidence Regarding Platelet Function Testing in Complex PCI
Numerous randomized and observational studies have investigated the potential role of PFT-guided antiplatelet treatment for the optimization of clinical outcomes of patients subjected to PCI.24,29–31,43–54 Supplementary Table 1 provides an overview of the relevant studies in this field. Smaller randomized and non-randomized studies suggest PFT-guided treatment tailoring improves clinical outcomes.51–54 Nonetheless, major randomized trials have failed to confirm a benefit from a PFT-guided treatment escalation approach whereas a PFT-guided de-escalation approach was found to be non-inferior regarding the primary endpoint of net clinical benefit (cardiovascular death, MI, stroke or BARC bleeding grade ≥2) compared with potent platelet inhibition for 12 months after PCI for ACS.24,28–31
C-PCI patients (e.g. those with multivessel PCI, LM PCI, saphenous vein graft PCI, bifurcation PCI, lesion type B2/C or three or more stents) are represented in these studies to varying degrees (mostly poorly), and relevant data are not reported in some of them (Supplementary Table 1). Moreover, only a few studies have reported subgroup analyses of the primary endpoint for some procedural variables related to PCI complexity but not for the subgroup of C-PCI patients in total (Supplementary Table 1). Consequently, extrapolation of the results of these studies to C-PCI patients is questionable.
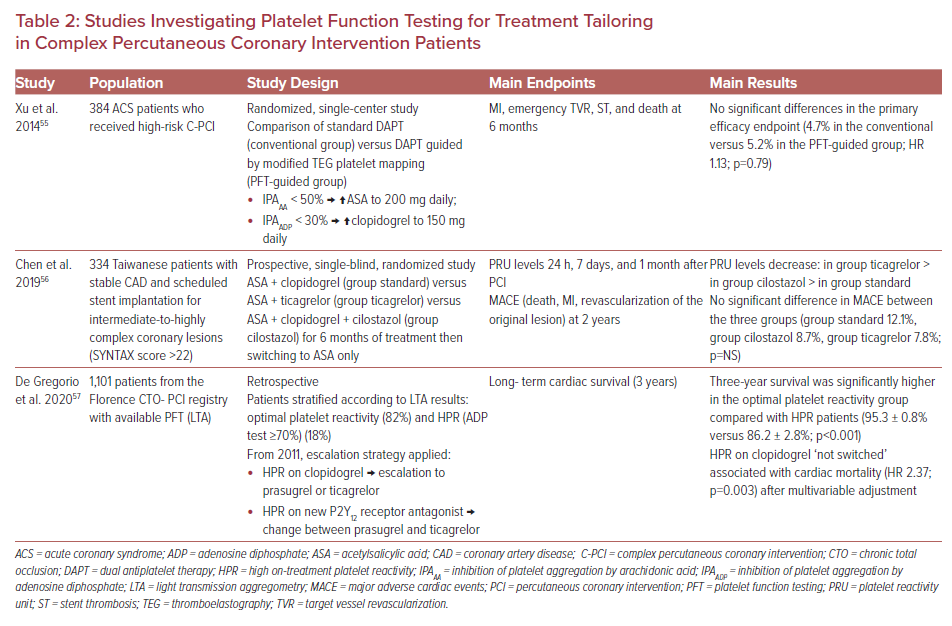
To have more robust evidence regarding the role of PFT in C-PCI, dedicated clinical trials enrolling exclusively patients subjected to high-risk interventions are required. However, relevant studies to date are scarce and suffer the limitations of small sample size or non-randomized design.55–57 The design and main results of these studies are presented in Table 2.
In a randomized, single-center study, Xu et al. failed to show any improvement in the primary ischemic endpoint by using a guided DAPT escalation strategy, using modified TEG platelet mapping in ACS patients who underwent C-PCI.55 Similarly, no significant difference in MACE was reported by Chen et al. by the administration of more potent antiplatelet therapy (aspirin plus ticagrelor, or aspirin plus clopidogrel and cilostazol) compared with standard treatment (aspirin plus clopidogrel) in stable CAD patients scheduled for C-PCI, in spite of achieving a greater platelet reactivity unit decrease with the more intense antiplatelet treatment. This study did not investigate the guided strategy, but implies that more pronounced platelet inhibition does not confer additional benefit in stable CAD C-PCI patients.56 Finally, in a retrospective study including 1,101 patients from the Florence CTO-PCI registry, HPR on clopidogrel ‘not switched’ was associated with increased cardiac mortality (HR 2.37; p=0.003) after multivariable adjustment, compared with an LTA-based treatment escalation strategy in HPR patients who underwent CTO PCI.57
Evidence Regarding Genotyping in Complex PCI
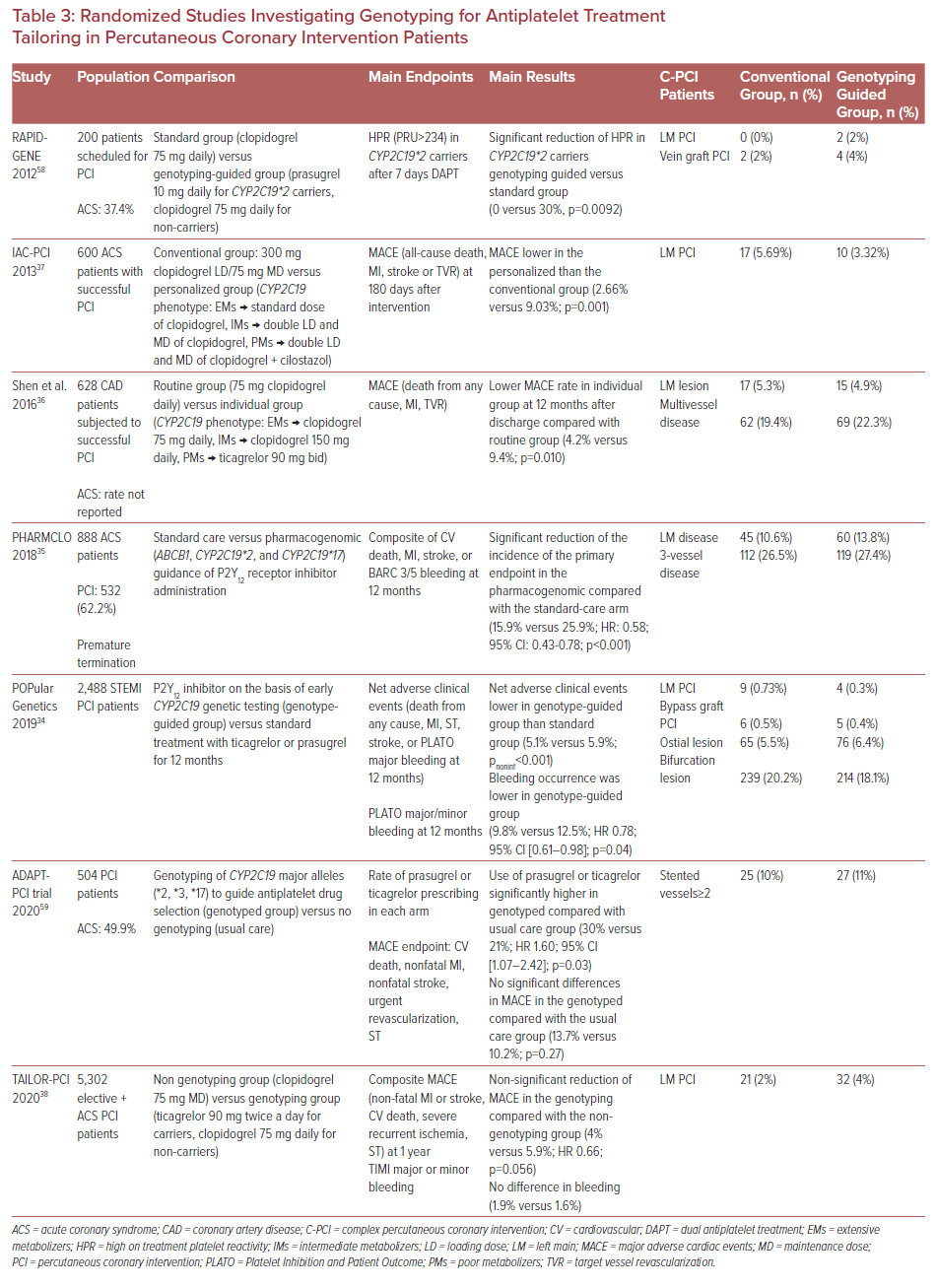
As with PFT, no studies regarding genotyping have been conducted exclusively for C-PCI. Table 3 contains a summary of the randomized studies investigating the role of genotyping (CYP2C19) for antiplatelet treatment tailoring in PCI patients.34–37,58,59
Although some small randomized studies have shown clinical benefit, the recent large-scale, randomized TAILOR- PCI study questioned the benefit from point-of-care, genotype-guided, anti-platelet therapy (ticagrelor 90mg twice daily for carriers and clopidogrel 75 mg daily for non-carriers) compared with routine care (clopidogrel 75 mg as directed) in patients undergoing PCI (electively or for ACS).34–38 The reduction of MACE (non-fatal MI or stroke, cardiovascular death, severe recurrent ischemia or stent thrombosis) at 1 year in the genotyping compared with the non-genotyping group was marginally non-significant (4% versus 5.9%; HR 0.66; p=0.056). No significant difference in thrombolysis in MI (TIMI) major or minor bleeding classification was noted as well (1.9% versus 1.6%).
Nevertheless, the results of this study show signs of benefit from the genetically guided antiplatelet therapy, since approximately one-third fewer adverse events occurred in the genetically guided treatment group compared with the standard treatment group. Notably, a prespecified sensitivity analysis allowing for multiple events per patient favored the use of genotype-guided therapy over the conventional approach in CYP2C19 LoF carriers (HR 0.60; 95% CI: 0.41–0.89; p=0.01).
Moreover, a post hoc analysis found a nearly 80% reduction in the rate of adverse events in the first 3 months of treatment among patients who received genetically guided therapy. This finding may be clinically relevant, considering that ischemic events are more frequent during the first 30 days after PCI in this population and potent P2Y12 inhibitor therapy may be beneficial during this early period. Bleeding is more pronounced during later period with potent P2Y12 inhibitors.
However, it should be noted that the representation of C-PCI in these studies was limited (Table 3). Therefore, it is not known to which extent their results are relevant to the C-PCI group of patients.
Conclusion
Taking into consideration the increasing performance of C-PCI procedures worldwide along with the associated high ischemic risk, refinement of antiplatelet treatment strategies is becoming imperative for this special group of patients.
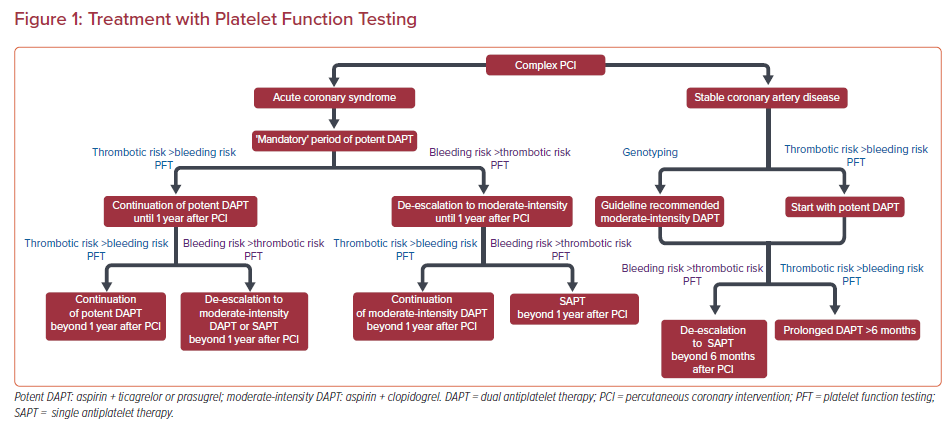
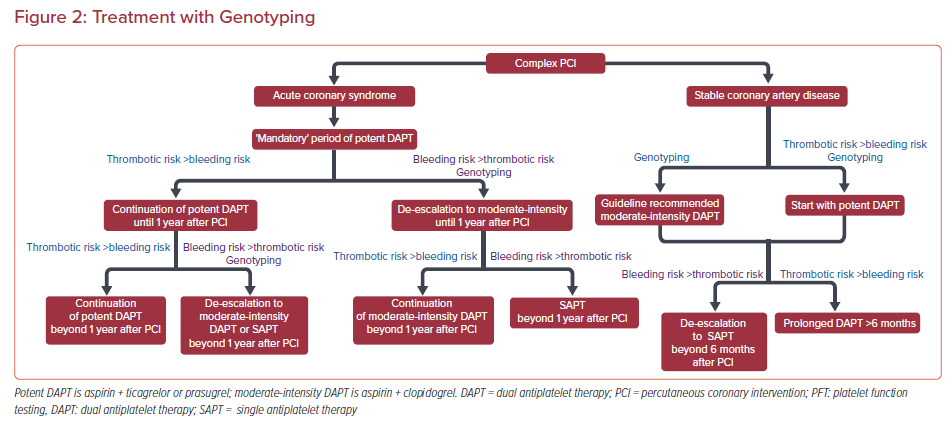
Tailoring DAPT potency and duration is a reasonable approach and PFT/genotyping may serve as valuable tools in this attempt (Figures 1 and 2). To date, large-scale randomized clinical trials have failed to show any clinical benefit from routine monitoring of platelet function in PCI patients. However, since C-PCI patients are mostly under-represented in these studies and subgroup analyses are limited, it remains unclear whether C-PCI patients would benefit from a tailored strategy guided by PFT.
Similarly, results from randomized studies are not convincing so far regarding the utility of genotyping results for treatment escalation after PCI. However, as with PFT studies, it is not known to which extent these results can be extrapolated to C-PCI patients. A patient-level meta-analysis, including C-PCI patients enrolled in PFT/genotyping studies, could potentially provide further insights into this field of research. Nonetheless, the heterogeneity in the design of conducted studies will definitely affect its reliability.
Dedicated randomized studies enrolling exclusively C-PCI patients in different clinical settings (such as stable CAD and ACS) are warranted and will elucidate the role of treatment tailoring based on PFT and genotyping in this group of patients. Notably, the establishment of a universal definition for C-PCI is required and will facilitate future research efforts.
Until more robust evidence becomes available in this controversial field, clinicians should follow the guidelines and adopt a PFT/genotyping-guided approach for treatment tailoring selectively, according to their clinical judgement, in specific C-PCI patients where balancing ischemic and bleeding risks is challenging (Figures 1 and 2). Noteworthy, the results of PFT and/or genotyping should always be evaluated in combination with the patient’s clinical, procedural and socioeconomic parameters to optimize antiplatelet treatment planning.
Click here to view Supplementary Material.
Clinical Perspective
- Patients with complex percutaneous coronary intervention (C-PCI) constitute a special PCI subpopulation at increased ischemic risk.
- Antiplatelet treatment in C-PCI patients remains controversial.
- Tailoring antiplatelet treatment in C-PCI patients is a reasonable approach and platelet function testing (PFT)/genotyping might serve as valuable tools in this.
- Existing evidence from clinical studies in this field is limited.
- Dedicated studies for C-PCI patients are warranted to elucidate the utility of antiplatelet treatment tailoring based on PFT and/or genotyping in this context.