Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in women, accounting for nearly one of every three deaths in women in the US annually.1 Peripheral artery disease (PAD) is a significant marker of systemic arteriosclerosis associated with increased all-cause, cardiovascular (CV), and cerebrovascular mortality, but it remains largely underrecognized and undertreated among women.2–4 Despite the increasing awareness of gender-specific risk factors and treatment disparities in CVD, much of the emerging understanding of PAD in women has not been applied to routine practice, where PAD is still perceived as a male-dominated disease.4,5 In 2022, the American Heart Association released a PAD National Action Plan to urge healthcare professionals to recognize the treatment gaps that remained among the population and women with PAD, and to find ways of providing care more sensitive to minority populations and women with PAD.6 In this review, we discuss sex- and gender-based differences in the epidemiology and risk profile for PAD, the presentation of PAD in women compared with men, and sex- and gender-based disparities in the diagnosis and management of PAD. For this discussion, we focus solely on atherosclerotic PAD involving the lower extremities, including the aortoiliac and distal branches, and exclude disease affecting other non-coronary arterial beds and non-atherosclerotic causes of PAD.
Epidemiology
According to the Global Burden of Disease Study from 2019, the global prevalence of PAD has increased by more than 25% from 2010 to 2019.7 This increase was primarily driven by countries with high social demographic index, such as the US and Denmark.7,8 The overall prevalence of PAD in the general population remains slightly higher in lower-middle-income countries (LMICs) than in high-income countries (4.32% versus 3.54%), especially in younger adults aged <45 years.9 However, among older populations, there remains a higher prevalence of PAD in high-income countries than in LMICs (21.24% versus 12.04% in adults 80–84 years in high-income countries versus LMICs, respectively).9
Compared with men, the prevalence of PAD among women was notably higher in the Global Burden of Disease Study, increasing from 1,686 cases per 100,000 women to 1,973 cases between 1990 and 2019; a 17% increase.7 In comparison, the prevalence among men rose from 778 cases per 100,000 men to 962 cases during the same period.7 Overall, the prevalence of PAD among women aged >40 years is estimated to be between 3–29%; however, even this is likely to be an underestimation. Most studies define PAD based on typical symptoms, evidence of PAD on diagnostic imaging, or a history of revascularization; however, these are less commonly seen among women, and women are underrepresented in PAD studies.3,10 Compared with men, women tend to experience more atypical lower-extremity symptoms and present with PAD later in life, the reasons for which we describe in this review.
The overall prevalence of PAD escalates with age, regardless of gender. Among adults aged >80 years, PAD is estimated to affect >20% of the population.11 However, among women, there is evidence that PAD prevalence increases with age to a greater degree than in men.12 In a 2010 US Census evaluation, more women aged ≥40 years had PAD than men.13 In a study by Diehm et al., although the prevalence of PAD was 12% for women and 17% for men in patients aged <70 years, in patients aged >85 years, women had a higher prevalence of PAD (39%) than men (27%).12 Furthermore, data from the National Health and Nutrition Examination Survey suggest that the highest prevalence of PAD is among non-Hispanic Black women aged >70 years (25.3%).2 Collectively, these data suggest that the gap in PAD prevalence between women and men tends to widen with age.
Clinical Presentation of Peripheral Artery Disease
The classic clinical presentation for PAD consists of intermittent claudication, but as much as 40% of patients have atypical presentations. Women have higher rates of atypical, subclinical, and asymptomatic PAD, with the majority of women presenting asymptomatically (defined as no symptoms with an ankle-brachial index [ABI] <0.9).14 Compared with men, women are more likely to present asymptomatically and less likely to report symptoms of intermittent claudication.15 Women diagnosed with PAD were significantly more prone than men to present with no leg pain symptoms (12.9% versus 9.4%, respectively).16 Furthermore, atypical symptoms in women can be misinterpreted as arthritis, neuropathy, or spinal stenosis because of their prominence in this population.8,14 Additionally, by the time women are symptomatic, they are older, have more comorbidities, and have a more advanced and complex disease progression, leading to higher mortality rates and adverse outcomes in women.10,15,17 Once diagnosed, women have twofold higher mortality than men.18
Data suggest that women with PAD suffer more from depression, have poorer overall health status, and have an increased risk of morbidity and mortality than men.19,20 Women have also been shown to have a higher functional and psychosocial impairment with PAD than men. Women diagnosed with PAD lead less functionally independent lifestyles with higher levels of physical impairment.10,15,17 This manifests as shorter initial and absolute claudication distances, slower age-adjusted walking speed, slower 6-minute walk distance (6MWD), more complex (multilevel) disease, and lower quality of life scores compared with men.10,14 Such differences were present despite similar ABI values between men and women.15 In a study by Dreyer et al., physical and mental health status were determined upon PAD diagnosis and 12 months later.21 Women had significantly poorer mental and physical health status at diagnosis and 12 months later, despite experiencing a similar magnitude of change in both during the first 12 months after diagnosis, thus demonstrating that gender is an independent determinant of poorer baseline and longer-term health status.21 Additionally, women aged <65 years diagnosed with PAD have a fourfold higher risk of depression than men.1 However, there may be aspects of social determinants of health attributed to such differences, since women with PAD are often non-white, avoid medical care because of cost, live alone, and have low physical and social functioning compared with men.10,22,23 Ultimately, the delay in diagnosis in women resulting from a either a lack of symptoms or presence of only atypical symptoms has been shown to result in the development of more severe and advanced disease, including chronic limb-threatening ischemia (CLTI) in women compared with men.8,14,24,25
Diagnosis
The diagnostic evaluation usually starts with standard ABI testing as a noninvasive tool that can be used to diagnose and monitor the condition. It is inexpensive and minimally time-consuming. In addition, it has been found to have a high level of specificity (83.3–99%) and accuracy (72.1–89.2%), but different levels of sensitivity (15–79%) for an ABI ≤0.90 in detecting ≥50% stenosis.26 A single study demonstrated that men had worse specificities in both ABI and toe-brachial index testing, yet even the authors stated that this finding “should be taken with precaution since multivariate analysis did not show any association [regarding diagnostic accuracy of ABI between men and women] and male gender is overrepresented in the meta-analysis.”27 Regarding noninvasive imaging approaches, including duplex ultrasound, CT angiography, and magnetic resonance angiography, no studies have shown sex- or gender-based differences in imaging accuracy for PAD.28 The most significant barrier in diagnosing PAD in women likely remains clinician bias and a failure to recognize atypical symptoms.28
The rationale for screening asymptomatic patients to detect PAD is based on the potential to prevent PAD progression and reduce CVD risk.17 Guideline recommendations from several organizations suggest screening for asymptomatic PAD with ABI in higher-risk individuals. In the 2016 American College of Cardiology/American Heart Association guidelines, ABI screening was suggested for the following high-risk groups: adults aged >65 years, adults aged ≥50 years with risk factors for atherosclerosis or family history of PAD, and adults aged <50 years with diabetes and any additional risk factor for atherosclerosis.17 Similarly, the most recent Society of Vascular Surgery recommendation was against screening adults with no risk factors, history, signs, or symptoms, but screening with ABI was considered “reasonable” for adults with higher risk factors: current smoking, diabetes, age >70 years, abnormal pulse examination, or other established CVD.29 The US Preventive Service Task Force has concluded the evidence to be insufficient to make a recommendation regarding ABI screening.30
As demonstrated by these guidelines, effective diagnosis and initiation of the evaluation require identifying symptoms and risk factors first. As previously mentioned, women tend to be asymptomatic or have atypical symptoms and usually present with more significant functional impairment, such as walking difficulties and progressive functional decline.17,31 A higher prevalence of atypical symptoms may be one of the reasons there exist disparities in screening for PAD in women compared with men.17
Risk Factors for Progression of Peripheral Artery Disease
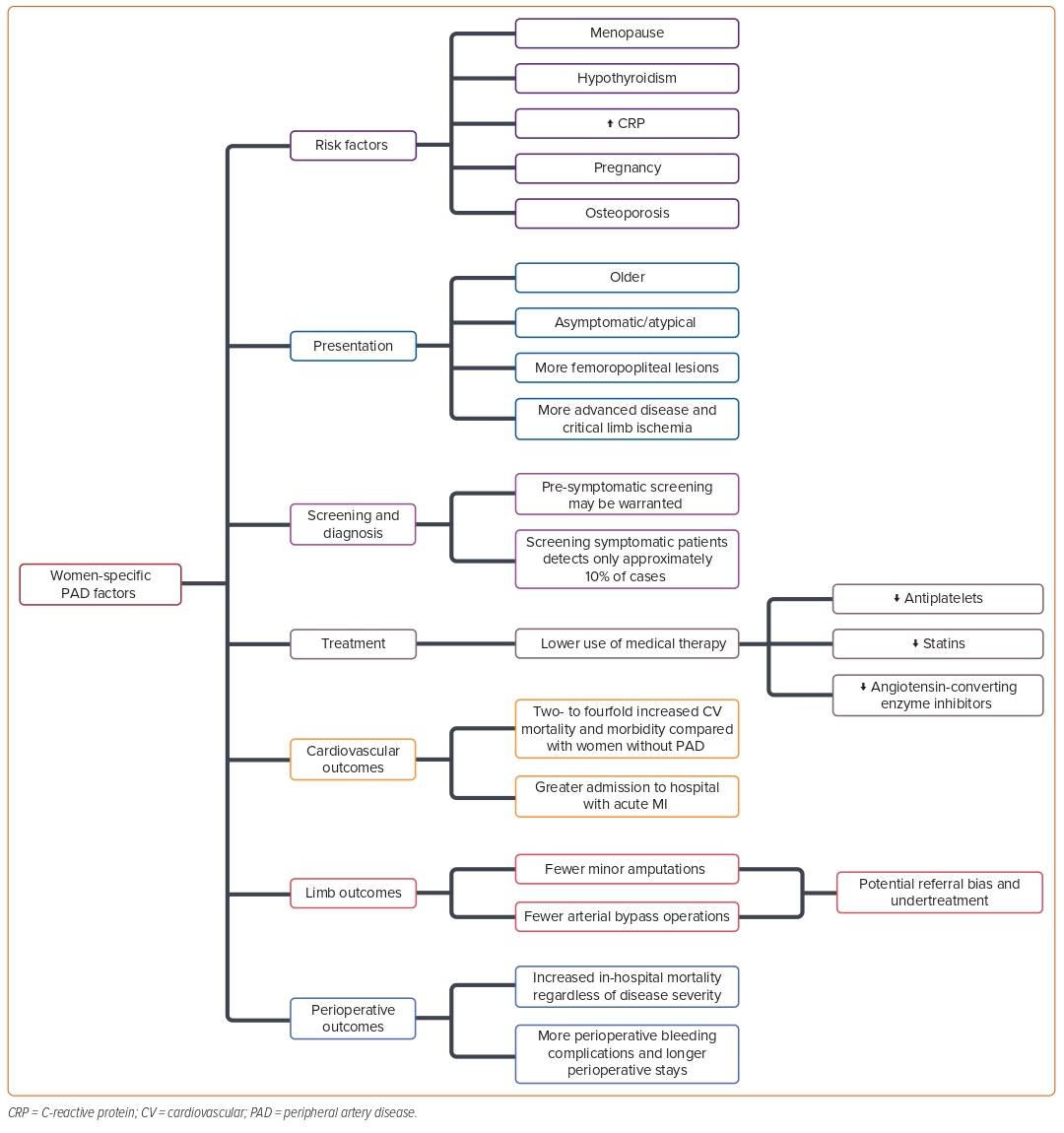
There are limited data on the sex and gender differences in PAD risk factors and disease progression. In this section, we summarize the current state of the evidence regarding these differences and the pathogenesis of PAD. A summary is presented in Figure 1.3
Diabetes and Obesity
Diabetes is a well-recognized risk factor for PAD and is second only to smoking in its attributable risk.28 Although women with diabetes have a greater gender-specific risk for overall atherosclerotic disease, the evidence for gender-specific diabetes risk for PAD is less clear.28 Among women with diabetes and PAD, a higher HbA1c level was associated with a higher prevalence of severe PAD based on an ABI of <0.6 in a study by Aronow et al.; however, the same association was noted among men.32 Diabetes and dysglycemia have been shown to increase the risk of intermittent claudication by fourfold in women,18 and – when comparing the gender-specific risk of claudication associated with diabetes – there is an increase of 8.6-fold in women compared with 3.5-fold in men.18,33
The relationship between obesity, defined by a BMI of ≥30 kg/m2, and PAD remains debatable.34 Although it is widely observed that obesity and PAD often occur in the same patients, studies have failed to consistently demonstrate a positive association between PAD and obesity. Several studies have even demonstrated a better overall CVD prognosis in patients with overweight and mild obesity, a phenomenon often described as the obesity paradox.28,35 When evaluated based on metrics of abdominal fat deposition as opposed to BMI, several studies have indicated an association between central adiposity and PAD, especially in women.36,37 In a study by Lu et al. evaluating the association of PAD with two specific measures of abdominal obesity, waist-to-thigh ratio and waist circumference, although waist-to-thigh ratio was associated with PAD risk in both women and men, waist circumference was associated with PAD only in women.37
Pregnancy
Women who experience complications during pregnancy are at increased risk of developing premature CVD and atherothrombotic occlusive vascular disease, including PAD.24,38 In a retrospective population cohort study of 1 million women who were free of CVD before pregnancy, those who had a maternal placental syndrome (defined as a composite exposure of preeclampsia, gestational hypertension, placental abruption, or placental infarction) had a significantly higher risk of developing PAD than those who had not (adjusted HR 3.0, 95% CI [1.9–4.8]).24 In a smaller case-control study by van den Bosch et al., the presence of angiographically confirmed aortoiliac disease (i.e., distal aorta, common iliac artery, internal or external iliac artery) was found to increase the risk of miscarriage threefold, independent of the presence of other vascular and obstetrical risk factors.38 Additional research indicates that hypertension in pregnancy is an independent risk factor for PAD decades after pregnancy, even when adjusting for multiple contributing risk factors.39 DeCarlo et al. showed that, among all those who gave birth at an integrated health system in Massachusetts between 1998 and 2020 between the ages of 18 and 50 years, pregnancy was associated with an increased risk of acute peripheral arterial events, especially when complicated by preeclampsia.40
C-reactive Protein
Serum C-reactive protein (CRP), an inflammatory marker, has proven to be an independent marker of the extent of atherosclerosis in patients with coronary, cerebrovascular, and peripheral arterial disease. Among PAD patients, serum CRP has been shown to correlate with disease severity – as measured by ABI – and future adverse events, including death and coronary, cerebral, and peripheral arterial events.4 Rein et al. demonstrated that systemic inflammation is high in PAD, with elevated CRP and white blood cell counts, concluding that inflammation plays a role in the initiation and progression of PAD.41 CRP levels associated with the development of PAD are essential to highlight, as women have been shown to have higher levels of CRP than men, even after adjusting for comorbidities, age, and hormone status.16 It is not fully understood why women have more elevated CRP levels than men, but some potential theories include the quantity and distribution of body fat influencing inflammation more in women than men,underlying causes of stress and inflammation (such as socioeconomic factors), and estrogen.42–44 A multicenter, population-based, case-control study of young women found that elevated CRP levels increased the likelihood of PAD almost fourfold.45
Additional research has further reinforced such findings. For example, women participating in the Life Line Screening program had higher CRP levels and a higher prevalence of PAD than men.46 Neither higher CRP levels nor conventional CVD risk factors explained women’s excess prevalence of PAD. In both the Multi-Ethnic Study of Atherosclerosis (2006) and the Dallas Heart study (2005), women had significantly higher CRP levels than men, despite adjustment for estrogen use, BMI, and other variables.46–48
Chronic Kidney Disease
In patients with chronic renal insufficiency, there may be a higher prevalence of PAD in younger women compared with age-matched men.14 This is particularly interesting because women are more likely to be diagnosed with chronic kidney disease than men and have poorer outcomes.49 Adverse pregnancy outcomes can also increase the risk of kidney disease. For example, preeclampsia occurs in 3–10% of pregnancies and is associated with acute kidney injury, an increased risk for hypertension, and chronic kidney disease.50
Osteoporosis
Osteoporosis has been previously associated with the presence of atherosclerotic CVD overall, with PAD being highly prevalent in women when compared with age- and race-matched non-osteoporotic post-menopausal women.51,52 In one study, Mangiafico et al. compared bone mineral density in osteoporotic patients with and without PAD, demonstrating that osteoporotic patients with PAD had lower bone mineral density scores than those without PAD.51 Additionally, in a prospective observational cohort from the Rancho Bernardo study, the prevalence of osteoporosis was significantly higher in women with PAD compared with women without PAD.53 During a mean follow-up of 4 years, women with PAD were found to demonstrate significantly higher rates of bone loss compared with women without PAD.
Like PAD, the prevalence of osteoporosis increases with age and is growing because of the constant aging of the population. In addition, women are more at risk of developing osteoporosis than men because of the hormonal changes during menopause that directly affect bone density, such as a decrease in the level of estrogen, which is vital for bone health.54 However, these associations might be because of osteoporosis being a confounding factor rather than a contributing factor, and more studies are needed to understand any such association fully.
Menopause and Hormone Replacement Therapy
The menopausal transition is associated with detrimental changes in lipids and lipoproteins, glucose and insulin metabolism, and body fat distribution, leading to increased rates of hypertension and diabetes and a deterioration of vascular function.55 Menopause is another sex-specific risk factor for delayed PAD presentation in females. Postmenopausal women lack the vasoprotective properties of estrogen, which include lowering LDL, raising HDL, promoting antioxidative defense, and decreasing cytokine-induced inflammation.5 Overall, CVD risk in females increases after the cessation of ovarian function at menopause.5,55 Collectively, these factors lead to a deterioration of vascular function and increase the risk of symptomatic PAD development after menopause.55
Data showing the protective effects of estrogen have led to studies looking at whether exogenous estrogen through hormone replacement therapy (HRT) can decrease atherosclerosis and reduce menopause-associated CVD.56–58 Nevertheless, data regarding the effects of HRT in post-menopausal women have been contradictory.56–58 More recently, larger randomized controlled trials from the Women’s Health Initiative have found no difference in PAD outcomes with conjugated equine estrogens versus placebo, albeit with a nonsignificant trend towards an increase in peripheral arterial events.56 These data suggest that, although HRT does not appear to reduce the odds of developing PAD in postmenopausal females, taking exogenous estrogen may instead potentially increase the risk of morbidity from vascular events.56 Other large, well-designed trials, including the Heart and Estrogen/Progestin Replacement study, have also not demonstrated a benefit from using HRT for PAD or CVD.57,58
Conversely, several observational studies have suggested that HRT may be beneficial over the long term, even though the CV event risk may be higher over the short term.16 The Rotterdam study suggested that HRT given for a year or more is associated with a decreased risk of PAD among postmenopausal women, with those who used HRT for >1 year showing a 52% decreased risk of PAD and the risk of PAD was not affected by HRT use for ≤1 year.20 Another study found lower degrees of atherosclerosis by ultrasound of the carotid arteries, aorta, and iliac arteries in 40 users of combined replacement therapy compared with never users.6 Current recommendations from the North American Menopause Society support using HRT for women aged <60 years or who are within 10 years of menopause onset and have no contraindications, among whom the benefit–risk ratio is favorable based on the available evidence.59 However, for females who initiate HRT more than 10 years from menopause onset or beyond the age of 60, the benefit–risk ratio appears less favorable because of a greater risk of CVD, including coronary artery disease (CAD), stroke, and venous thromboembolism.59
Hypothyroidism
Hypothyroidism has been linked to dyslipidemia and atherosclerotic disease, including PAD, but there are contradicting data on its role as a sex-dependent risk factor. Some data, such as Powell et al., showed that women with PAD were found to have a significantly higher thyrotropin level than controls.60 Elevated thyrotropin levels were strongly correlated with elevated serum cholesterol levels.60 However, Mazzeffi et al. also found a gender difference in the association of hypothyroidism and PAD, but they reported a negative association for women.61 This negative association in women, but a positive association in men, was speculated to be because of greater recognition and treatment of hypothyroidism in women than in men.61 In contrast, a large prospective trial did not find any difference in PAD risk between patients with subclinical hypothyroidism and euthyroidism over a 4-year follow-up period.14
Medical Management
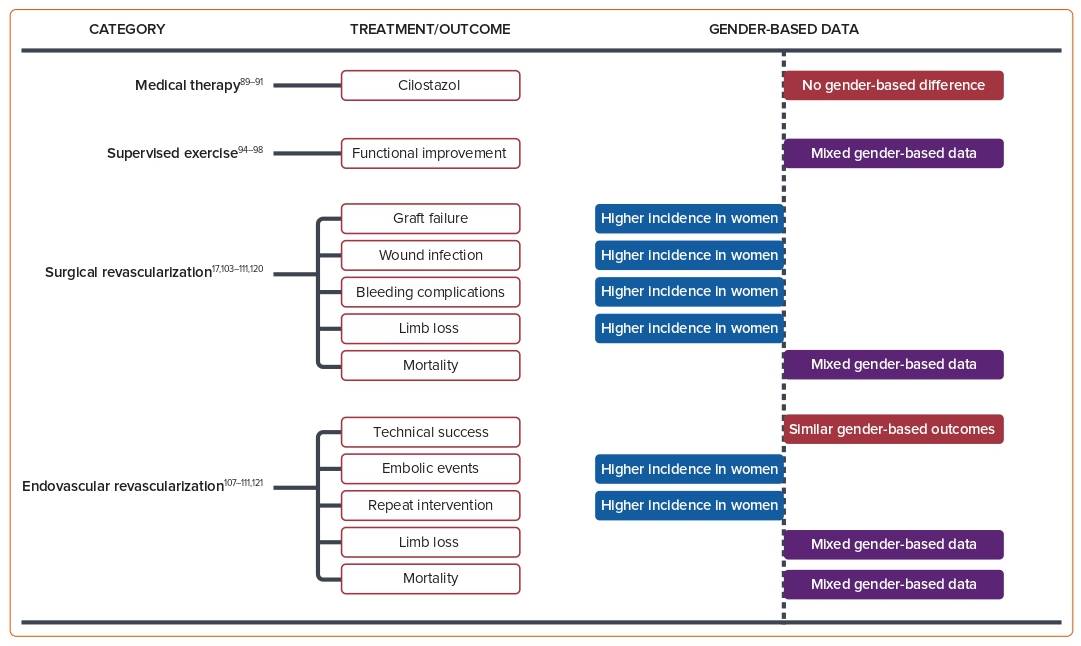
The primary goals of medical management in PAD are to improve limb symptoms and preserve functional status, and to reduce other CV or cerebrovascular events, including mortality. Current guidelines for medical management options include smoking cessation, treatment of co-existing medical conditions (e.g. lipid-lowering and antihypertensive agents), antithrombotic therapy, and structured exercise. Surgical management and revascularization, including endovascular and percutaneous options, are reserved for patients with persistent symptoms despite maximally tolerated medical therapy.62 Figure 2 summarizes medical and surgical therapy outcomes in women.63
Lifestyle interventions, such as weight loss, physical activity, and reduced fat intake, help to reduce the risk of disease progression from glucose intolerance to diabetes and help to improve CV risk factors.64 Tobacco cessation is also critical, and is associated with improved outcomes after surgical and endovascular interventions.65,66
Smoking Cessation
Smoking is a leading risk factor for PAD, and smoking cessation is essential for reducing morbidity and mortality in patients with PAD.67,68 Overall, the burden of smoking-related PAD deaths has declined modestly in both men and women over the past three decades.69 Although women have a lower overall rate of smoking than men, the relative CV risk from smoking is especially pronounced in women, and the PAD-specific risk is at least similar, if not potentially higher, in women.28,46,70,71 In an analysis from the Women’s Heart Study, even former smokers continued to have a threefold greater risk for developing PAD compared with never smokers.72
Lipid-lowering Strategies
Lipid-lowering therapies have been shown to reduce major vascular events, including stroke and coronary events (defined as MI, coronary death, or revascularization), independent of baseline cholesterol levels.73,74 Statins have also been shown to improve limb function and reduce limb events.73,75 The randomized FOURIER trial revealed that evolocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, versus placebo on top of statin therapy significantly reduced CV events risk with a significant absolute risk reduction, and lowering LDL with evolocumab reduced the risk of major adverse limb events.76 Despite evidence that lipid-lowering strategies significantly reduce adverse events, women are less likely to be treated as aggressively men.77
Sigvant et al. reported that lipid-lowering therapy was more common among men than women with an OR of 1.3 (range 1.1–1.5).78 This unbalanced allocation of healthcare resources to men has also been reported by Stramba-Badiale et al.79 The REGARDS study revealed that 65% of individuals without any vulnerabilities have access to statin therapies compared with only 45% of individuals with four or more vulnerabilities, suggesting that patients with multiple simultaneous vulnerabilities, in particular Black women and those without health insurance, experience the most significant disparities in statin use.80
Antihypertensive Therapies
Antihypertensive agents, including angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), have been shown to reduce the risk of major CV events in atherosclerotic disease and are recommended secondary prevention strategies in patients with PAD.17 A post hoc analysis from the HOPE Study, which compared ramipril with placebo, demonstrated that patients with PAD benefited from ramipril with a significant reduction in primary CV events.81 Later, the ONTARGET trial revealed that telmisartan, an ARB, was equivalent to ramipril in preventing the risk of death, MI, and stroke in patients with vascular disease or high-risk diabetes and was associated with less angioedema; however, the combination of telmisartan plus ramipril was associated with more adverse events without an increase in benefits.82 In the REACH Registry, beyond the overall finding that patients with PAD were less likely to achieve optimal risk factor control – including blood pressure control – than patients with CAD or stroke, women, in particular, were less likely to get access to antihypertensive therapies and achieve target blood pressure than men.83
Antithrombotic Therapies
Two major randomized trials, the 2017 COMPASS Trial and 2020 VOYAGER Trial, have evaluated the role of rivaroxaban (at a dose of 2.5 mg twice daily) plus aspirin in PAD management.84,85 The COMPASS trial found that the combination of rivaroxaban plus aspirin had better CV outcomes (including MI, stroke, and death) than aspirin alone, but was associated with an increased rate of major bleeding events.84 Rivaroxaban alone did not result in better CV outcomes and was associated with an increased rate of major bleeding events.84 In VOYAGER, which evaluated the efficacy and safety of rivaroxaban in patients with PAD who had undergone lower extremity revascularization, low-dose rivaroxaban plus aspirin was associated with a significantly lower incidence of acute limb ischemia, major amputation, MI, stroke, and CV death compared with aspirin alone.85 However, although both trials suggested a net potential benefit of antithrombotic therapy in PAD patients, fewer than 25% of participants in both trials were women.84,85
Antiplatelet Therapies
Antiplatelet agents, primarily aspirin and P2Y12 inhibitors, including clopidogrel and ticagrelor, have been used in patients with PAD to prevent limb-related adverse events and major vascular outcomes.86–89 The CLIPS trial evaluated the effect of low-dose aspirin (81 mg) for secondary prevention in a population that included asymptomatic patients in addition to patients with symptomatic PAD (i.e. Fontaine stages 1 and 2) and demonstrated a significant benefit for aspirin with a 64% relative risk reduction (RRR) in major vascular events in the aspirin-treated group.86 A subsequent meta-analysis revealed that aspirin failed to reach statistical significance regarding the RRR of CV events in patients with asymptomatic or symptomatic PAD.89 In the CAPRIE trial, clopidogrel (75 mg once daily) was compared with aspirin (325 mg once daily), revealing a 23.8% RRR in the primary composite endpoint of ischemic stroke, MI, or vascular death in the clopidogrel group.87 Lastly, the EUCLID trial evaluated clopidogrel versus ticagrelor in symptomatic PAD patients, and the results revealed that ticagrelor was not superior to clopidogrel regarding the reduction of CV events.88
In a study by Sigvant et al., antiplatelet therapies were more commonly used overall among men than women, with an OR of 1.6 (range 1.3–2.1).74 Pâquet et al. reported similar treatment rates of antiplatelets between genders; however, men significantly more frequently used combination medication regimens (including antiplatelets, ACE inhibitors, and statins).90 Similar results were reported by Lanéelle et al., who showed that a combination of an antiplatelet agent plus an antihypertensive and statin was less frequently prescribed in women (29.2% versus 53.9%; p=0.038).91
Cilostazol
Cilostazol is an effective medical therapy for the treatment of leg symptoms and for improving functional status in PAD.17 Across two separate Cochrane meta-analyses of large randomized clinical trials, cilostazol improved walking distance in patients with stable intermittent claudication; however, there are insufficient data regarding whether cilostazol reduces all-cause mortality and adverse CV events.92,93 Although there is an increased incidence of adverse effects, such as headache, palpitations, and diarrhea with cilostazol, the effects are generally mild and tolerable.93 Currently, only one study has demonstrated as part of a subgroup analysis that cilostazol significantly increased walking distance in both men and women.94
Exercise Therapy
Supervised exercise therapy is now recommended as a first-line therapy for symptomatic treatment of claudication as a class 1a recommendation.62 A large body of evidence, including 37 identified randomized trials and seven systematic reviews or meta-analyses, supports the use of supervised exercise therapy to improve both limb symptoms and walking performance in patients with PAD.95 A Cochrane review included eight randomized clinical trials in patients with intermittent claudication, showing that supervised exercise therapy improved maximal treadmill walking distance compared with a non-supervised exercise therapy regimen.96 Of note, only one observational study by Lundgren et al. performed gender analysis, and there was no significant influence on walking performance between men and women.97 In trials comparing supervised exercise with pharmacological therapy, the most significant increase in walking distance was observed in the combination of exercise programs and pharmacological therapies. Studies also compared supervised exercise therapies with home-based/no-feedback exercise. In a retrospective analysis, Manfredini et al. showed that a hospital-prescribed, structured home-based walking program was equally effective at improving treadmill walking ability and 6MWD in both women and men with symptomatic PAD with a similar degree of program adherence.98 Gommans et al. revealed that women with intermittent claudication benefit less during the first 3 months of supervised exercise therapy and have lower walking distances after 12 months of follow-up compared with men.99 Women with symptomatic PAD, particularly those with diabetes, were shown to respond poorly to exercise rehabilitation, and they might need more intense exercise or exercise in combination with other therapies.100
The mechanism of underlying gender differences in exercise programs is unclear. Microvascular gender differences have been reported in patients with symptomatic PAD, where a lower hemoglobin oxygen saturation of the calf muscles was observed during exercise program in women compared with men.101,102 Further investigations of sex- and gender-specific outcomes in response to exercise and underlying mechanisms are warranted.
Revascularization
Representation in Revascularization Studies
Historically, the evidence showing the efficacy of revascularization for PAD is based mainly on studies where most participants have been male. Among PAD revascularization trials that reported gender, women accounted for 32% of randomized trial participants (range 0–54%).103
Feinglass et al. historically reported a significant gender disparity in patient selection for lower extremity revascularization, where men were twice as likely to be selected as candidates. At the same time, no significant baseline differences for the prevalence of limb salvage indications or other comorbid conditions were noted.103 Egorova et al. later analyzed 2.4 million PAD-related inpatient admissions and found that women had a lower rate of procedural revascularization during inpatient hospitalization for PAD (46% versus 54%).25 The reason for gender disparity in procedure selection is currently being studied; potential contributing factors are that women with symptomatic PAD present at an older age and have more advanced disease.104,105 In addition, women tend to have smaller caliber arteries that may result in more technical difficulties and have the potential for worse procedural outcomes, such that operators are less likely to offer PAD interventions.106
Revascularization Outcomes
Women are usually older than men and have more advanced disease and CLTI when they present to have lower extremity revascularization. Procedural outcomes for women could be negatively affected because of these factors.104,105 The data regarding outcomes based on gender show mixed results. Ferranti et al. analyzed the association between gender and peripheral vascular intervention outcomes for intermittent claudication and CLTI.107 They observed higher rates of access site complications in women but no evidence of gender disparity in reinterventions, major amputation, or survival rates at 1-year follow-up.107 Wang et al. revealed that after revascularization, women had significantly inferior short-term outcomes, such as 30-day mortality, amputation, and early graft thrombosis, but similar long-term outcomes, such as survival and limb salvage, compared with men.108 A large observational study of the Blue Cross Blue Shield of Michigan Cardiovascular Consortium PV registry revealed that compared with men, women who underwent lower extremity peripheral vascular interventions showed a higher rate of vascular complications, transfusion needs, and embolism rates.109 However, no differences were observed for in-hospital death, MI, stroke, or transient ischemic attack. Women had more technical success than men (91.2% versus 89.1%; p=0.014), but because of a higher complication rate, the overall procedural success rates were similar in men and women (79.7% versus 81.6%; p=0.08).109
Studies from the Korean K-VIS ELLA registry reported that, based on a large population of patients with PAD undergoing endovascular treatment, women had higher rates of complex lesions, procedural complications, and limb-specific adverse events, along with higher rates of death, cardiac events, and major amputation than men.110 In a post hoc secondary analysis of the PREVENT III Trial, Nguyen et al. discovered that Black ethnicity and female sex were risk factors for adverse outcomes after vein bypass surgery for limb salvage, including graft failure and limb loss, with Black women being particularly high risk.111 Regarding longer-term outcomes, a study by Hussain et al. identified that women were less likely than men to undergo minor amputation and arterial bypass surgery,4,112
Conclusion
Women with PAD are more likely to present asymptomatically or with atypical PAD symptoms, experience more functional impairment, and have more advanced disease compared with men. Although gender-specific risk factors may contribute to the disease’s increased morbidity in women, creating awareness among patients and medical professionals will likely lead to better understanding, increased support, and more targeted and aggressive treatments for women with PAD. Such interventions would lead to reduced adverse CV outcomes and enhanced functional status and quality of life for women with PAD.