The Current State of Sudden Cardiac Arrest
Sudden cardiac arrest (SCA) in the US accounts for an estimated 180,000–300,000 deaths per year.1 Autopsy studies show that about half of sudden cardiac death patients have no specific findings on autopsy, while the other half has specific findings, such as MI, heart failure, or pulmonary embolism.2 Although national educational and prevention campaigns have increased the awareness of SCA and out-of-hospital cardiac arrest (OHCA), the survival after an OHCA remains low at 9.1%.2,3 In the chain of survival, bystander initiation of cardiopulmonary resuscitation (CPR) increases the 30-day survival rate to 10%.1 The effect is magnified when defibrillation with an automated external defibrillator (AED) is performed early by lay first responders. In fact, defibrillation by lay first responders was found to have the highest impact on survival (median 53%) when compared with professional emergency first responders (28.6%).1 In addition to survival, other benefits of bystander efforts include decreased rates of neurological damage and nursing home admissions.4 This improvement increases progressively as the arrival of emergency medical services is delayed. The increase in survival underlies the undeniable potential of pursuing innovation in cardiac arrest.
In this review, we describe new innovations to address unmet needs in the identification, treatment, and prevention of SCA and highlight technological advances such as machine learning and new AED designs in new public health models to reach the right person at the right time. We also discuss the priorities for reducing disparities in OHCA towards equitable interventions for communities at risk. Improving outcomes requires a combination of technological advancements and public health objects to propel the future of diagnosing and treating SCA.
Gap Analysis
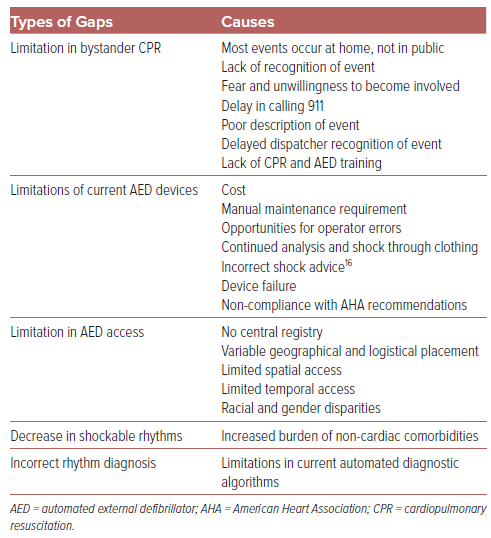
Table 1 lists several unmet factors limiting the outcomes of SCA. The lack of AED use during the moments after a person’s cardiac arrest is associated with limitations in AED devices themselves, as well as public health efforts. Because most ventricular tachyarrhythmias occur in public spaces, our efforts should focus on the use of AEDs outside the hospital.5 While the availability of these life-saving devices has increased over the past two decades, current devices are expensive (up to and greater than $3,000 per unit, plus an additional $150–400 for ongoing battery and electrode pad replacement).6 They are designed to be mounted in permanent holding locations, limiting their overall accessibility. Due to the necessity of manual maintenance, AEDs are often improperly maintained or neglected, leading to high rates of AED malfunction and failure. Device failures that have been reported to the Food and Drug Administration (FDA) include not powering on, powering off unexpectedly, failure to complete rhythm analysis, and failing to deliver a shock.7 Because of improvements in preventing and treating coronary artery disease, the incidence of shockable rhythms, particularly ventricular fibrillation, has decreased.8
The use of AEDs relies on the bystander to look for and find a nearby device. There is no existing map of AEDs in the US, and there is no central registration of the devices, although there is an ongoing approach to create one through the Dynamic AED Registry.9 Approximately 1 million AEDs have been purchased in the past 20 years, but the precise location of each device is not known.10 In addition, placement of AEDs in businesses that close after business hours can limit access during evenings and weekends. As many as 62% of OHCAs occur in the evenings, nights, and weekends, resulting in a 53% reduction in AED coverage.11–13 While most AEDs are placed in open access cabinets, occasionally they are restricted and require personnel assistance, which can lengthen the time to access an AED.14
Even when AEDs are available during an OHCA, a lack of awareness and education of AEDs prevents their effective use. The general population is found to have as high as an 80% failure rate when compared with a 10% failure rate in trained physicians including anesthesiologists and surgeons.15 Successful defibrillation requires turning on the AED, placing AED pads on appropriate locations of a patient’s bare chest, waiting for analysis, prompting with voice and light, charging, and pushing the shock button while standing clear. Common mistakes include not removing the patient’s clothing, not placing pads correctly, and touching the patient while defibrillating.15 Up to 72% of errors associated with AED use are caused by the operator.16
Emerging Innovations to Address SCA
Improving Patient Identification with Electronic Medical Record Analytics
Advanced computational approaches such as machine learning have emerged to potentially improve the identification of patients shortly before a cardiac arrest. Lee et al. developed the Deep learning-based Early Warning System (DeepEWS) to predict cardiac arrests that occur in the hospital.17 The algorithm learned the relationship between vital signs and cardiac arrest and outperforms traditional track-and-trigger systems such as the Modified Early Warning Score (MEWS) with a higher specificity (87.0% versus 79.9%).18 Although the DeepEWS has fewer variables than the MEWS, it has the advantage of being able to interpret the vital signs based on one another, such as interpreting an elevated heart rate differently depending on the body temperature.18
In patients with hypertrophic cardiomyopathy, machine learning was used to identify lethal ventricular arrhythmias for risk stratification to determine the need for ICD implantation. Using an electronic health dataset that involved 93 variables from echocardiography, cardiac MRI, Holter monitors, and EKGs, the investigators were able to demonstrate 12 new predictors of ventricular arrhythmias, including global longitudinal strain. By combining multiple machine learning methods, the new model had a higher area under the receiver operating characteristic curve (AUC) than the baseline model (0.83 versus 0.80).19 Similar predictive analytics by reviewing clinical diagnoses from the medical record have also been performed for patients with QTc interval prolongation to identify those at highest risk for torsade de pointes.20
Among patients with heart failure and left ventricular dysfunction, machine learning with Cox proportional regression modeling was used to develop a prognostic model for heart failure survival models that was found to be more accurate than the traditional Seattle Heart Failure Model, with an 11% improvement in AUC.21 The limitations of such analyses using medical record data include the quality and quantity of the dataset that trains the algorithms. Even with perfect methodology, a dataset that does not contain adequate predictors will not produce a strong machine learning algorithm.22
Artificial Intelligence and Machine Learning Approaches to Cardiac Rhythms and High-risk EKG Phenotypes
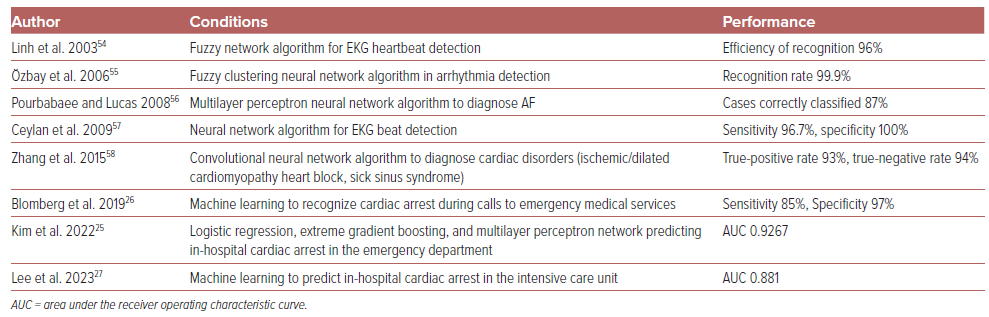
EKGs and vital signs are particularly rich in quantitative data, making them well-suited for machine learning. Over the years, a number of EKG classification schemes has been proposed and implemented for use to improve the reporting of shockable rhythms. A few such machine learning approaches include frequency domain analysis, gradient pattern detection, waveform shape matching techniques, and neural networks to predict EKG rhythm classification.23 Multiple studies test and compare the variety of machine learning methods for performance accuracy (sensitivity, specificity, AUC), and they report performances as high as 95–99% accuracy (Table 2).23–27 In fact, some research has aimed at determining whether algorithms can even exceed the accuracy (precision, recall, F1 score) of cardiologists.28
A common classification approach is to extract selected features of the EKG and to base classification on those features. Extracted features include time intervals such as PR interval, QRS, RR interval, and QT interval; amplitudes of P wave, QRS, or ST segment; shapes of P wave, QRS, and T wave; directions of the P wave, QRS, and T wave; and irregularity. These extracted features are submitted to a classifier, which in turn classifies the heart signal based on the extracted features. The classification results are then used to determine whether the detected cardiac rhythm is a good candidate for defibrillation shock therapy.24,28–30
Among the challenges in using machine learning for EKG interpretation is the abundance of individual features of EKG waveforms compared with a limited number of EKG training data. Often, the number of extracted features needs to be reduced or selected to be generalizable. Different techniques such as dimensionality reduction and feature selection optimize different feature combinations for a smaller subset of features to use in the classification.24 Combining multiple techniques improves learning further. For example, an algorithm combines a convolutional neural network with multiple input channels (stand-alone EKG, shockable, and non-shockable signals) with secondary learning (a boosting classifier) that improves accuracy to 99.25%.31
Machine learning can learn the EKG features not only of a particular rhythm, but also of a particular disease. A fully convolutional neural network approach reached the performance of human cardiologists to detect MI.32 Machine learning also has the ability to extract underlying EKG features in cases, such as hypertrophic cardiomyopathy. Primary T wave inversion not secondary to QRS abnormalities was found to be associated with a higher risk of sudden cardiac death.33
Innovations to Improve Bystander CPR
Lay persons who learn CPR by a novel kiosk approach with an on-screen practice with feedback outperform those who learn CPR by video only.34 In the Netherlands, a simple method was tested to improve bystander defibrillation rates. A network of trained volunteers was available and notified via a text message alert system in the event of cardiac arrest. It involved a text message alert system for trained volunteers in the community. When a text message alert resulted in a trained volunteer responder, the recorded rhythm was more often shockable (59.9% versus 4.5%), suggesting a reduced response time, and survival to hospital discharge increased (27.1% versus 16% alive at discharge; OR 1.95; 95% CI [1.15–3.33]).35 Another application, Heartrunner, dispatches volunteer responders who accept an alert, although a small randomized clinical trial of the application did not find a significant difference in AED attachment given that the majority of AEDs were attached by lay volunteers.36
New Automated External Defibrillator Technologies
Multiple studies have used machine learning to detect shockable rhythms in AEDs.31,37–39 We have recently described the creation of a novel convolution neural network (CNN) to diagnose a shockable rhythm from a single-lead EKG in next-generation, miniaturized AEDs. The AED was designed for personal use in community settings.40 Our approach was to develop a 26,000 single-lead EKG dataset consisting of publicly available and patient care EKGs, which was used to train, validate, and test the performance of a CNN that conformed to the American Heart Association (AHA) criteria for performance. A six-layer CNN was developed, with five convolutional layers and one fully connected layer, and performance was tested on the hardware of the AED device itself. In both internal and external validation analyses, this CNN was found to have high accuracy even with varying levels of noise applied to EKGs to mimic muscle artifact, and to have better performance compared with individual physician EKG readers. Intended for use in the field during OHCA, the neural network can generate an output in 383 milliseconds. The input is an EKG strip 7 seconds long, therefore, added together, the AED has a total time of 7.383 seconds to reach a decision. Together, these results are adequate to improve the chain of survival of cardiac arrest.
Other advances include mobile applications that create an online map via crowdsourcing to enable a quick mobile search for the nearest AED when the time arises.10,41 One mobile application, PulsePoint, includes an AED registry added by users and a community of individuals who sign up for notification by location to nearby emergencies requiring CPR.41,42 In Stockholm, Sweden, researchers tested AED-equipped unmanned aerial systems, or drones, that were dispatched and found to be faster than emergency medical services.43,44
Public Health Innovations
The Dynamic AED Registry is authorized by the FDA to catalog each AED with a 2D matrix code that records its location and status, which are tracked using a smartphone and passed to a confidential online database in real time. Users are encouraged to scan the code after using the AED, and then they answer preset questions.9
Novel AEDs with improved machine learning techniques should be incorporated into public health emergency medical services systems of care. Key performance measures include recording the use of AEDs and regular service checks.
Corti is a Danish company that created a machine learning framework that listens to emergency phone calls, learning the words most often spoken in critical illness and the background noise accompanying it. When it detects cardiac arrest, it signals the emergency operator to advise the caller to begin chest compressions. While dispatchers recognized a cardiac arrest at an average of 54 seconds, Corti recognized it in 44 seconds. In addition, the model predicted cardiac arrest with a higher sensitivity as compared with medical dispatchers (84.1% versus 72.5%).26
The AHA aims to improve the use of AEDs through public access defibrillation programs that ensure that AEDs and trained lay rescuers are available. After primary training, 90% of volunteers retain competency in AED skills for up to 1 year.45 One of the major goals of the AHA is to improve public access defibrillation programs. Key recommendations for effective sites include planned and practised responses, ongoing training of lay rescuers, and links with local emergency medical services.46
In a landmark trial, the use of trained volunteers was capitalized upon by using a mobile-phone positioning system to dispatch them when an emergency occurred. This increased the rate of bystander-initiated CPR from 48% to 62%.47
Equitable Approaches to Reduce Disparities in OHCA
Unfortunately, there are significant disparities in OHCA.48 There is a gender disparity in CPR, with more men (45%) receiving public bystander CPR than women (39%).49 Black and Hispanic communities have significantly lower rates of bystander resuscitation, thought to be secondary to lower rates of CPR training.50 Although there are similar bystander CPR rates, Asian individuals have lower survival than white individuals.51 It is unknown whether disparities in comorbidity or post-resuscitation care exist for Asian versus white patients. There is also significant geographic variation among emergency medical service agencies, including response times and length of resuscitation.52 Systemwide improvement is needed to improve these disparities, focused on funding, training, and measuring success metrics.53
Proposing a Data-driven and Technology-enabled SCA System of Care
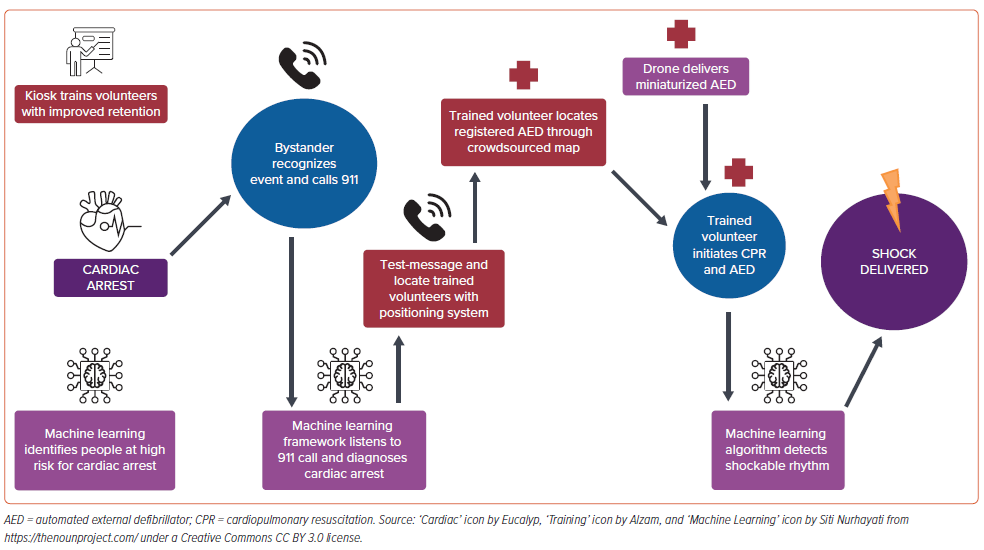
Despite the life-saving capabilities of defibrillation with AEDs, these devices are left unused during cardiac arrest. We propose a data-driven, technology-enabled system of care to improve outcomes of SCA (Figure 1). This involves machine learning algorithms to identify individuals at high risk, recognize emergencies, and diagnose rhythms. It requires new technology to make AEDs available where and when they are needed. Last, we need a public health framework to train volunteers and integrate the intelligence and innovation to save lives.