Postural orthostatic tachycardia syndrome (POTS) is a disorder that can present with various non-specific symptoms, although it is primarily characterized by symptoms of orthostatic intolerance lasting at least 3–6 months. Clinically, this condition is defined as:
- orthostatic intolerance associated with an increase in heart rate of 30 BPM (>40 BPM for adolescents) or a rate that exceeds 120 BPM within the first 10 minutes of standing;
- symptoms that occur with standing, including lightheadedness, palpitations, tremor, generalized weakness, and exercise intolerance; and
- the absence of orthostatic hypotension, defined as a fall of 20/10 mmHg in blood pressure with standing.1–3
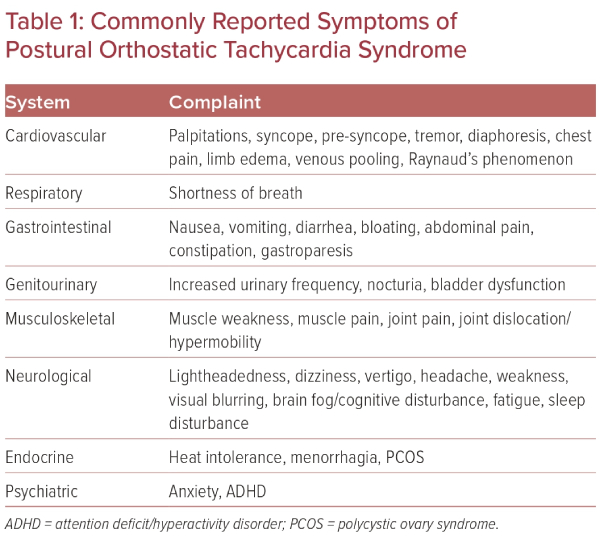
It is estimated that between 500,000 and 3 million Americans have POTS, which mainly affects women of childbearing age, with a 5:1 women to men predominance.2,4 Patients will commonly present with complaints of palpitations, fatigue, nausea, ‘brain fog’, lightheadedness, exercise intolerance, tremulousness, syncope, or near syncope, among other symptoms (Table 1).5
Diagnosis of POTS is often delayed due to a clinical presentation that includes a constellation of non-specific symptoms. These symptoms overlap with various other conditions, including anxiety and depression. A complete history and physical examination are critical components of diagnosing POTS, and should focus on the chronicity of symptoms, potential triggers, family history, patient diet and exercise history, and modifying factors.1 Because of the somewhat vague nature and broad range of these symptoms, patients often require many physician visits, possibly including multiple subspecialty referrals, before a diagnosis of POTS is confirmed.4 Unfortunately, many patients are dismissed by providers as having a psychosomatic origin to their symptoms, which leads to frustration with the medical system and doubt towards the care they receive.
Diagnosis of POTS requires ruling out of other conditions with similar presentations, including thyroid disorders, adrenal gland disorders, anemia, iron deficiency, and electrolyte abnormalities, among many others.6 Initial workup should include orthostatic blood pressure and heart rate measurements at 2-, 5-, and 10-minute intervals and a comprehensive physical examination including evaluation for lower extremity venous pooling and joint hypermobility.3 Adjunct testing includes tilt table testing, 24-hour ECG and ambulatory blood pressure and heart rate monitoring, implantable loop recorders, echocardiography, and exercise ECG.7 Treatment regimens are extremely patient specific and primarily focus on symptom improvement and gradual exercise tolerance.
Primary Postural Orthostatic Tachycardia Syndrome and Pathophysiologic Mechanisms
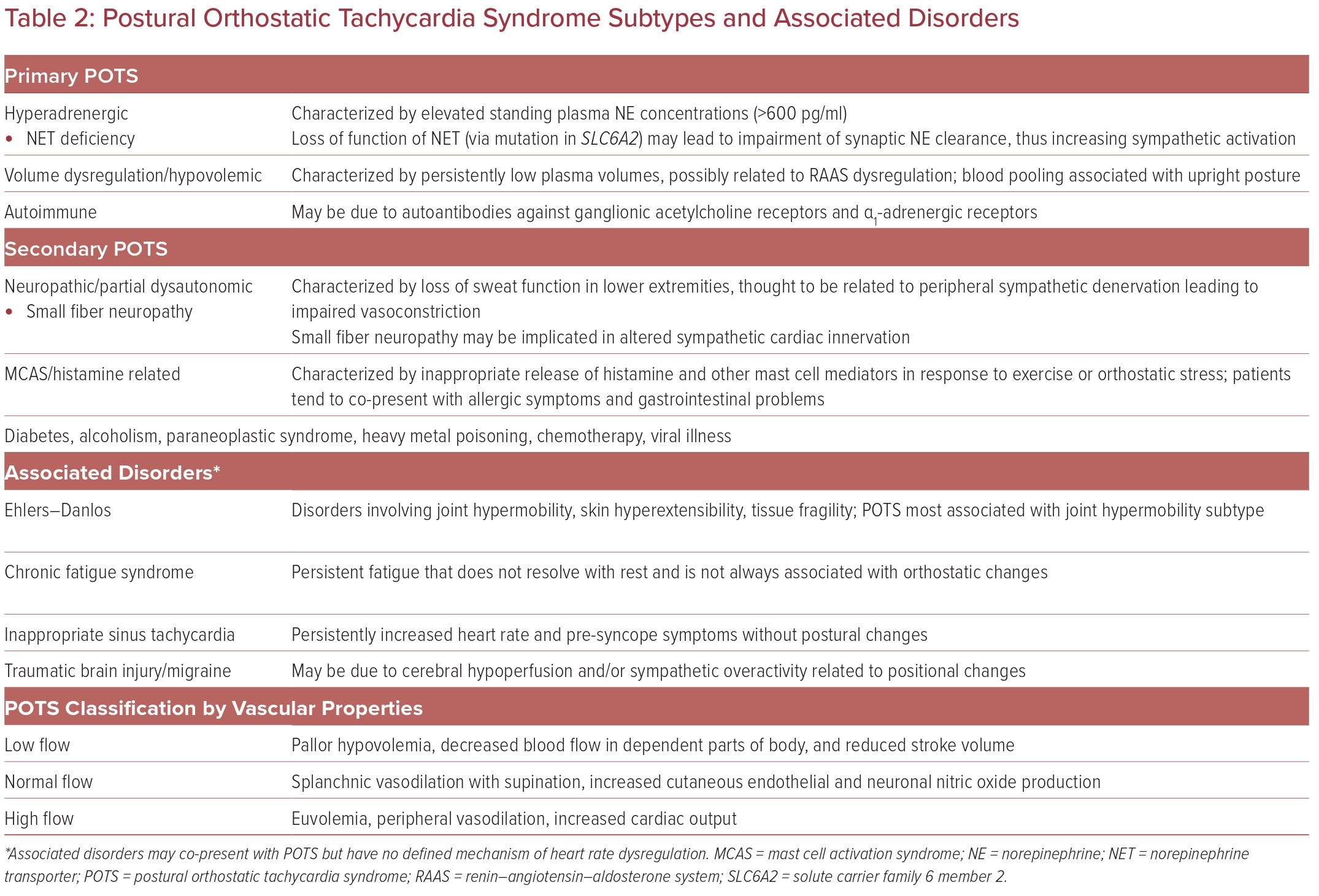
Although categorization of primary and secondary POTS is sometimes variable, we have identified three main mechanisms of primary POTS (Table 2). Although multifactorial in origin, one leading hypothesis of primary POTS pathophysiology is a dysregulated return of venous blood and subsequent unloading of baroreceptor signaling to increase sympathetic activation.8 Cardiovascular deconditioning may be implicated in POTS via cardiac atrophy and hypovolemia causing reactive tachycardia.9
Around 30–60% of POTS patients have symptoms consistent with the ‘hyperadrenergic POTS’ subtype, characterized by palpitations, tremulousness, and gastrointestinal (GI) symptoms.9 Patients with this subtype have elevated standing plasma norepinephrine concentrations causing increased sympathetic activation.3 This is thought to be due to a loss of function of the norepinephrine transporter, leading to impaired norepinephrine clearance from neuron synapses.
Autoimmune-related POTS may be related to the occurrence of POTS symptoms after certain insults or triggers, such as viral infection or stress.9 Patients with autoimmune-related POTS may also present with autoimmune disorders as comorbidities. The onset of POTS in these patients may be related to autoantibodies against ganglionic acetylcholine receptors and α1-adrenergic receptors, although no definitive pathways have been identified.10
Older classification systems use peripheral vascular properties to categorize patient presentations, termed low-flow, high-flow, and normal-flow POTS.9,11 Low-flow POTS is characterized by pallor of the extremities and decreased blood flow in gravity-dependent parts of the body. Normal-flow POTS may involve normal peripheral blood flow but splanchnic vasodilation when supine, redistributing blood flow by venous pooling. High-flow POTS describes increased cardiac output but inadequate peripheral vasoconstriction.
Importantly, there may be significant overlap between existing medical conditions and POTS.5,9,11,12 Discussion of various POTS associations and subtypes, along with current therapies and treatments, is the subject of this review.
Secondary Postural Orthostatic Tachycardia Syndrome and Associated Disorders
Various conditions have been associated with POTS. The associations vary but include concomitant conditions, similar proposed pathophysiologic mechanisms, common treatment modalities, and anecdotal experiences. Understanding these associated conditions can help improve care for patients with POTS and possibly shed light on underlying mechanisms to progress future endeavors in managing this complicated syndrome.
Mast Cell Activation Syndrome
Mast cell activation syndrome (MCAS), a subset of mast cell activation disorder (MCAD), is one of the conditions that can be associated with POTS and is characterized by either early or excessive mast cell activation and histamine release.13,14 Mast cells have a wide range of functions throughout the body, including inflammatory homeostasis, tissue repair, angiogenesis, nervous system function, and immune response.15 Biochemical inflammatory markers released by mast cells include histamine, prostaglandins, and tryptase.14 Atypical mast cell activity can lead to various symptoms, including, but not limited to, flushing, pruritus, urticaria, headache, nasal congestion, nasal pruritus, wheezing, diarrhea, constipation, throat swelling, angioedema, and hypotension.14
Although an exact pathophysiologic mechanism has not been identified, activated mast cells may produce circulating vasodilators such as histamine, leading to flushing and orthostatic intolerance as part of a hyperadrenergic POTS presentation.16 Vasodilation results in a compensatory sympathetic response with increased vascular resistance and tachycardia, ultimately leading to the release of norepinephrine and neuropeptide Y, which are thought to further activate mast cell degranulation and create a positive feedback loop. This hypothesis does not fit perfectly with the positional changes seen in autonomic dysfunction in POTS and MCAS but provides some speculation as to the possible relationship.16
Kohno et al. examined 69 patients referred to their medical center for POTS and assessed laboratory and clinical findings that may be associated with comorbid MCAS.14 They found that there was a relatively high number of MCAS symptoms and laboratory findings among POTS patients. Patients with laboratory values consistent with MCAS (particularly elevated prostaglandins and histamine markers) had a diverse set of symptoms compared with ‘typical’ POTS presentations, including allergy and GI issues, which further underscores the overlapping symptoms in these two patient populations.14 Very few studies have explored the resolution of POTS symptoms when treating MCAS. Weinstock et al. reported a single case report in a patient with MCAS, POTS, and small intestinal bacterial overgrowth who demonstrated marked improvement in POTS symptoms after treatment with low-dose naltrexone and intravenous immunoglobulin.17 Notably, this patient did not experience relief of POTS symptoms on the typical antihistamine regimen to manage MCAS, although anecdotally we have noticed clinical improvement in POTS patients with MCAS when using antihistamines or other MCAS-targeted therapies.
Hypermobile Ehlers–Danlos Syndrome and Hypermobility Spectrum Disorder
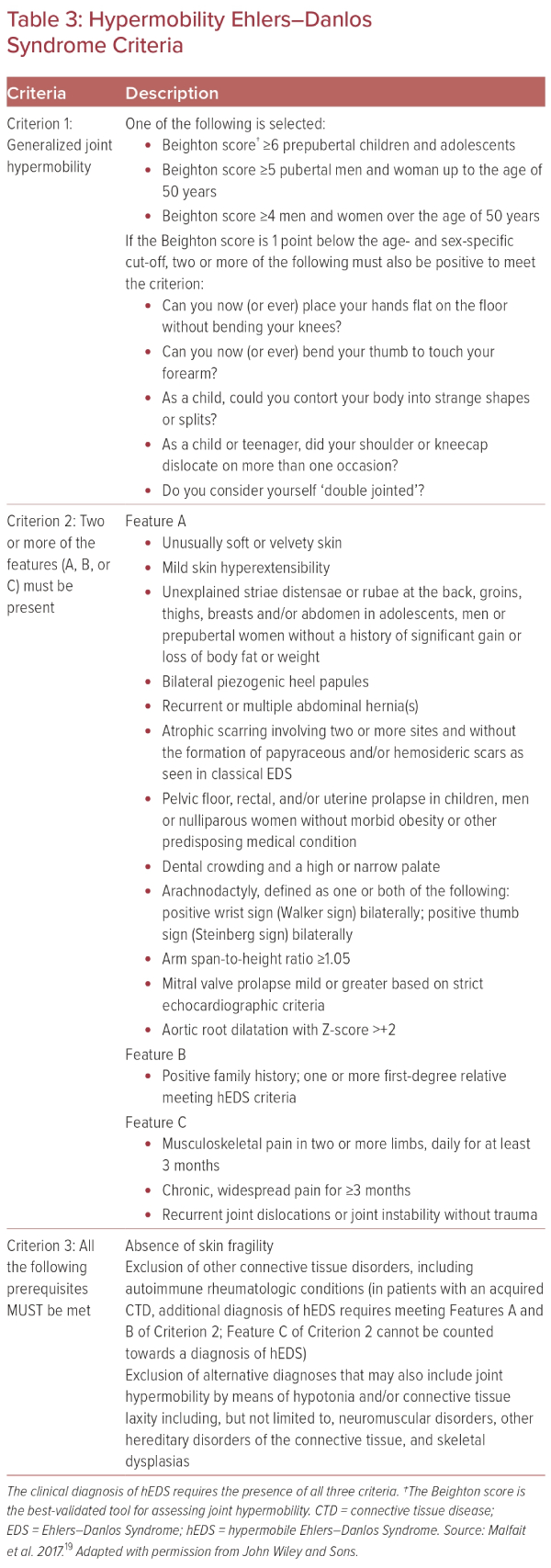
Ehlers–Danlos syndrome (EDS) is a group of inherited disorders that share common features, including hyperextensible skin, hypermobile joints, and fragile tissues. Among several subcategories of EDS, hypermobile EDS (hEDS), or Type 3 Ehlers–Danlos (EDS3), is most associated with POTS and is considered a less severe phenotype than classic EDS.9,18 Diagnostic criteria for hEDS (Table 3) are divided into three general criteria:
- generalized joint mobility;
- evidence of syndromic features, musculoskeletal complications, or family history; and
- exclusion of alternative diagnoses.19
Hypermobility spectrum disorder (HSD) is a similar diagnosis to EDS that fails to meet the same diagnostic criteria but is still subject to hypermobile joints and abnormal autonomic regulation.20
Pain and fatigue are common symptoms of hEDS and HSD, despite poorly understood mechanisms. These symptoms often overlap with those of chronic fatigue syndrome (CFS), fibromyalgia, and other pain disorders. Autonomic dysfunction/POTS is also often a component of hEDS and HSD, with symptoms, such as hypotension, orthostasis, dizziness, palpitations, exercise intolerance, and memory/concentration problems.18
Celletti et al. found that the frequency of POTS in 99 patients diagnosed with hEDS or HSD reached up to 50%.21 Peebles et al. evaluated a smaller cohort of 30 hEDS and HSD patients and found that HSD patients had a higher prevalence of POTS than hEDS patients (43% versus 7%, respectively) on tilt table testing, but not on active standing.20 It is thought that hypermobility disorders may lead to atypical connective tissue that composes the vasculature, leading to abnormal circulation with resultant sympathetic activation and orthostatic symptoms, although other studies have not demonstrated this association.22,23 α- and β-adrenergic hyper-responsiveness has also been implicated in hEDS cardiovascular autonomic dysfunction.23
Prior studies have suggested a connection between hEDS, POTS, and MCAS. Several small studies have associated elevated baseline tryptase levels with an autosomal dominant inheritance pattern.24 Lyons et al. identified germline duplications and triplications of the tryptase alpha/beta 1 (TPSAB1) gene, which encodes α-tryptase, in 96 patients in 35 families with elevated baseline tryptase levels and multisystem symptoms.24 Symptoms in these patients included flushing/pruritus (51%), autonomic dysfunction (46%), and congenital skeletal abnormalities (26%).24 Although none of these patients met the diagnostic criteria for POTS, MCAS, or hEDS, their symptoms fall into categories that can be attributable to each of these disorders. In addition, triplications in TPSAB1 were noted to be associated with higher baseline tryptase levels and more significant symptoms, relative to duplications.24 Although that was a smaller study with data comprising weak associations, it does serve as evidence for a possible underlying mechanism that may be further explored through continued research into these conditions.18,25 There are no targeted therapies for POTS patients with comorbid hEDS or HSD, and symptomatic treatment with multiple modalities is recommended to alleviate symptoms.23
Small Fiber Neuropathy and Complex Regional Pain Syndrome
Small fiber neuropathy (SFN) is the term given to conditions characterized by abnormal function of unmyelinated or thinly myelinated sensory fibers.26,27 These nerves are involved in various neurologic pathways and play a significant role in the autonomic nervous system.28 Symptoms resulting from SFN manifest as either sensory disturbances, autonomic dysfunction, or a combination of the two.27
SFN is felt to be caused by either excess firing or axonal degeneration of these small nerve fibers due to some underlying pathology, including immune-mediated, metabolic, toxic, and genetic conditions, although SFN in children and young adults is often thought to be inflammatory in etiology.28 One of the more common hypotheses for the underlying pathophysiology involves autoimmunity against various G-protein-coupled receptors (GPCR).29 GPCR autoantibodies have been shown to induce a POTS-like phenomenon in animal models, which resolved after clearance of the autoantibodies.27 In addition, some smaller studies reported finding GPCR autoantibodies in POTS patients.27
SFN is suspected to contribute to the underlying pathologies of many different conditions, including POTS, CFS, complex regional pain syndrome (CRPS), and fibromyalgia, among others, and overlaps considerably with CPRS.30 CRPS is a condition that typically develops after surgery or injury to a limb and is characterized by nerve dysfunction with sensory, motor, and autonomic neurologic abnormalities. CRPS was previously felt to be a localized disorder with regional symptoms, but more recent evidence has led to the belief that it is a systemic disorder with suggestions of generalized autonomic dysfunction.31 Although treatment of SFN is limited, studies have shown improved POTS symptoms in patients with SFN after treatment with intravenous immune globulin (IVIG) or subcutaneous immune globulin.32
Chronic Fatigue Syndrome
CFS, also known as myalgic encephalomyelitis (ME)/CFS, is a condition with significant overlap with POTS. However, neither the underlying etiology nor the pathophysiology of this condition are well understood. Autoimmune dysfunction, infection, and endocrine disorders have been suggested as being implicated in the primary underlying pathophysiology.27 CFS is characterized by symptoms of disabling fatigue, mental and physical postexertional malaise, pain, sleep disturbance, and cognitive impairment. The estimated prevalence of POTS in CFS is variable based on differences in orthostatic testing methods, but may reach rates as high as 50%.33
SFN has been proposed as an underlying mechanism of CFS, which may serve as a connection to neuropathic POTS and other similar disorders. Studies have reported on the prevalence of SFN in CFS based on skin biopsies and biomarkers, with SFN being detected in up to one-third of patients.27 This is felt to possibly contribute to dysregulation of microvascular tone by autonomic nerve fibers, impairing vasoconstriction and leading to venous pooling and reduced cardiac blood return.34 Higher laboratory markers of sympathetic activation have been noted in POTS patients with chronic fatigue as a complaint compared with POTS patients without fatigue.22 Although treatments specifically targeted for CFS patients with POTS are not clearly defined, the general recommendation for treating chronic fatigue in POTS patients includes many of the techniques described in Table 4, including, but not limited to, increased exercise, fluid intake, avoidance of aggravating factors, and electrolyte supplementation.33
COVID-19 Infection and Post-viral Postural Orthostatic Tachycardia Syndrome
COVID-19 infection has been associated with neurologic manifestations, including autonomic dysfunction similar to that seen in POTS patients, with postural tachycardia, orthostasis, and exercise intolerance.35,36 These symptoms have been noted to persist even beyond the period of acute infection, a condition known as long COVID.36 Although some patients recover fully from the initial infection, a significant proportion will have continued symptoms requiring prolonged therapy. These symptoms have been shown to persist 6–8 months beyond the period of acute infection, but may be present for even longer.37
Patients with long COVID may have some level of predisposing autonomic dysfunction prior to COVID-19 infection.37,38 Up to one-third of patients with COVID-19 dysautonomia have some type of cardiopulmonary abnormality prior to infection, which could serve to lower the threshold required for the virus to trigger an underlying autoimmune or inflammatory process.39–41 Long COVID may be implicated in similar mechanisms related to POTS, including neuropathologies, endothelial dysfunction, and blood flow abnormalities.42 Although COVID-19 brought the presentation of post-viral POTS into the spotlight, there have been several reports of autonomic dysfunction developing after viral illness even before the COVID-19 pandemic.43 Even though an exact mechanism has not been identified, it is suggested that autoimmune responses to viral illness may trigger POTS, with up to 50% of POTS patients reporting a viral illness preceding their symptoms.22
Migraine Headaches
Migraines are frequently reported among POTS patients, although the etiology of this association is not understood.44 Autonomic symptoms seen with migraines could result from activation of brain regions that control autonomic regulation, although activation of the nervous system may also play a role.30 Central sensitization, an increased response to nociceptors, is a key feature of migraines and has been demonstrated in POTS patients.30
Post-ganglionic sympathetic denervation and amplified sympatho–adrenomedullary axis activity are felt to be related to the pathophysiology of neurogenic and hyperadrenergic POTS, respectively. Sympathetic denervation may alter cerebral hemodynamics, resulting in reduced cerebral perfusion, which has been demonstrated in both migraines and POTS.30 Norepinephrine and other sympathetic mediators are noted to be elevated during migraines, suggesting increased activity of the sympatho-adrenomedullary axis.30
Post-concussive Syndrome
Orthostatic intolerance has been noted in patients with post-concussive syndrome. This is believed to have a similar pathophysiology to migraines, with cerebral hypoperfusion and/or sympathetic overactivity related to positional changes.45 Although most post-concussive symptoms are self-resolving over time, some patients can have prolonged symptoms. Orthostatic intolerance had a high prevalence in patients with prolonged symptoms, particularly when lasting for longer than 3 months.46 Interestingly, however, there are some discrepancies between patients with post-concussive syndrome and POTS patients.46 Post-concussive orthostatic intolerance lacks the strong predominance in women seen in POTS. In addition, despite some patients having prolonged symptoms, post-concussive orthostatic intolerance has a more favorable recovery timeline than POTS, with recovery in several months with or without therapy.47
Additional Associations and Postural Orthostatic Tachycardia Syndrome Mimickers
Common differential diagnoses for POTS include thyroid disorders and thyrotoxicosis, inappropriate sinus tachycardia, pheochromocytoma, anxiety, dehydration, infection, hypoadrenalism, and medication-induced tachycardia.5,48 The following conditions may present in tandem with POTS, although any mechanistic relationships are still under review.
Pelvic Congestion Disorder
A subset of POTS patients may present with pelvic congestion and venous compression disorders, particularly among patients who co-present with EDS.49,50 May–Thurner syndrome is one such disorder, in which the right common iliac artery compresses the left common iliac vein, causing pelvic venous congestion.50 Nutcracker syndrome is an obstruction of the left renal vein between the aorta and superior mesenteric artery.51 The ‘atypical’ form of nutcracker syndrome presents with fatigue, orthostatic intolerance, and dysmenorrhea and dyspareunia in women.51 Further research is needed to assess whether surgical intervention in these congestive disorders improves POTS symptoms in these patients.
Iron Deficiency and Anemia
Whether iron deficiency is specifically related to POTS or serves as a mimicker via diminished venous return is still under review.11 Dodson et al. found an association between restless legs syndrome, a condition often caused by iron deficiency anemia, and POTS.52 Disruption of nitric oxide activity from endothelial cells and subsequent postural vasodilation has been suggested as one mechanism for anemia-induced orthostatic intolerance.53 In our experience, women with MCAS complain of notably heavy menstrual cycles, and we recommend evaluating blood counts and iron studies in all patients presenting with orthostatic intolerance.
Spinal Cord Fluid Leak
Leakage of spinal cord fluid can cause orthostatic headaches due to intracranial hypotension.54 Prior reports have suggested a connection between spinal cord fluid leaks and POTS via autonomic dysfunction and possibly hypovolemia.55 There have been reports of co-occurrences of POTS and spontaneous intracranial hypotension.54,55 Disorders that lead to tissue weakening, such as EDS, may predispose POTS patients to spontaneous intracranial hypotension and migraines.55
Human Papilloma Virus and COVID-19 Vaccination
A controversial association between vaccination for human papilloma virus (HPV) and autonomic dysfunction has been suggested.56,57 Specifically, HPV vaccination has been associated with triggering many of the aforementioned diagnoses, including CRPS, POTS, SFN, and ME/CFS, and is thought to be related to autoimmunity and autoantibodies in these patients.57 Currently, no conclusive evidence suggests the development of POTS in HPV vaccine recipients, and the possibility of developing POTS is not a contraindication to receiving the HPV vaccine.56,58
Similarly, Kwan et al. reported increased odds of a new POTS diagnosis after COVID-19 vaccination in a cohort of 284,592 patients within 90 days of vaccination.35 POTS-associated diagnoses were 1.52-fold more likely after than before COVID-19 vaccination, although significantly less likely than in individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In addition to new diagnoses of POTS, they also identified increased odds of MCAS diagnoses, but lower odds of EDS diagnoses, after vaccination.35 Kwan et al. proposed that patients may have systemic immunologic responses to the SARS-CoV-2 spike protein, although to a lesser degree than actual viral exposure.35
Treatments and Therapies
There are various therapies available for the management of POTS. Although some of these therapies are recommended based on peer-reviewed evidence, there are additional medications that have been anecdotally found to be effective. With the range of clinical manifestations and associated conditions seen in POTS, the response to therapy varies widely, and finding the appropriate management strategy may require trial and error and should be individualized to each patient.
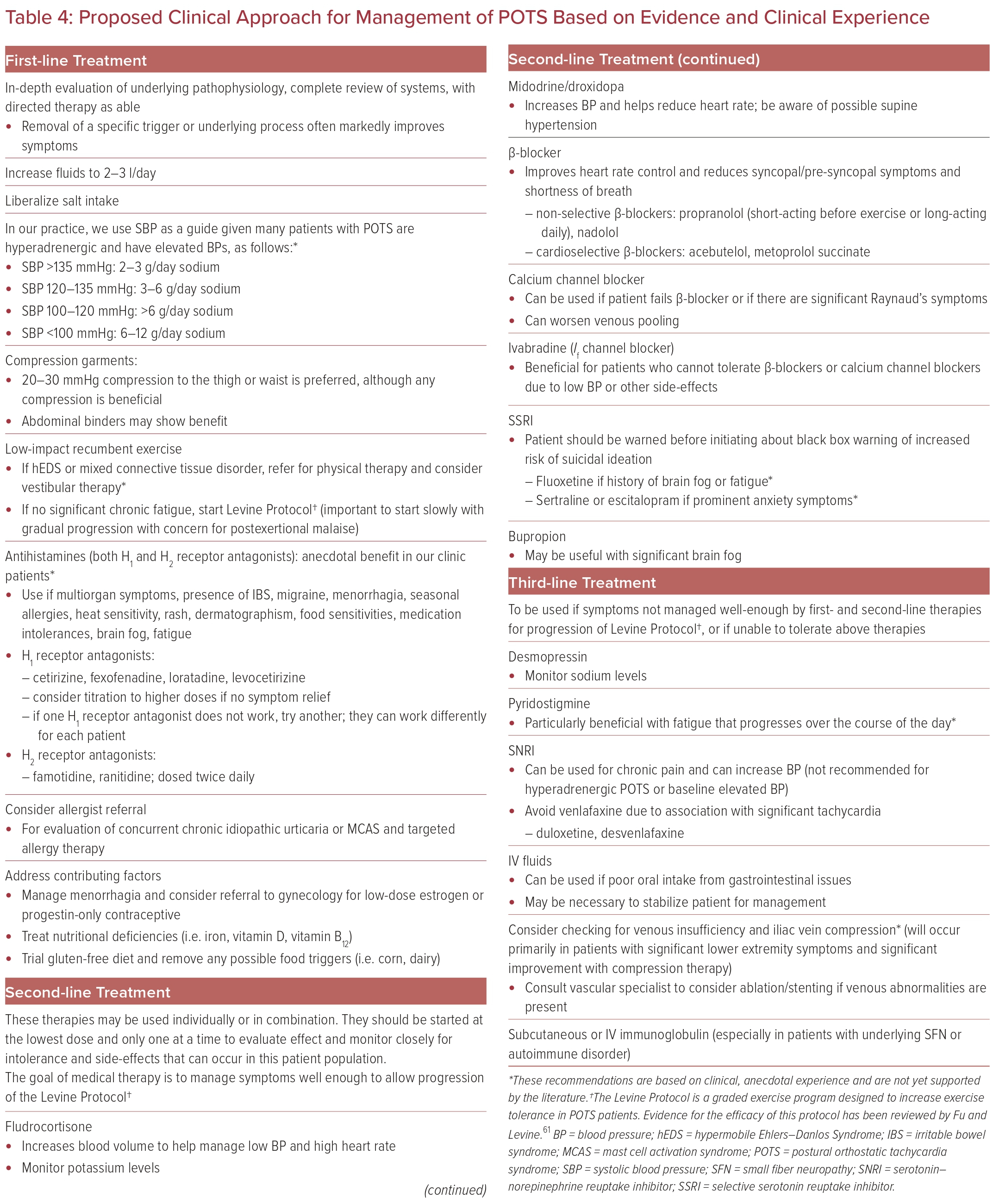
Special care should also be taken to not only treat POTS symptoms, but also to treat the potential underlying cause or associated condition the patient presents with. It is important for providers to know the first-line therapies that should be used in all POTS patients while also understanding other medications that are available and are best for specific clinical manifestations, as well as which to avoid based on their particular side-effects in this patient population (Table 4).
Wells et al. compared various treatment methods described for POTS patients, including intravascular volume expansion, vasopressor therapy, and heart rate reduction, to determine optimal treatment recommendations for treating POTS.59 These authors found moderate efficacy with each of the described treatments, but there was no outstanding therapy that alleviated symptoms. This may be due, in part, to limitations in randomized clinical trials and inconsistent questionnaires to evaluate efficacy. Below, we describe several non-pharmacologic, pharmacologic, and interventional therapies that may be considered in the management of POTS patients.
Non-pharmacologic Therapies
Exercise, increased salt (10–12 g/day) and fluid intake (2–3 l/day), and discontinuing medications that may contribute to POTS should be initiated in all patients diagnosed with POTS.6,8 Exercise regimens should start with maneuvers that do not require upright posture, such as rowing, swimming, and recumbent bicycles.9,60 Strength training improves physical deconditioning and small stroke volumes, and leads to improved quality of life in these patients. Physical maneuvers such as leg crossing, muscle pumping, and squatting may improve acute symptom onset.60 The Levine Protocol is a 3-month exercise program that has shown efficacy in lowering heart rates and improving cardiac output in POTS patients.61
Compression stockings are useful for decreasing lower extremity blood pooling and increasing venous return, although the stockings should reach at least the thigh for maximum benefit. In certain individuals, abdominal binders may also be beneficial by reducing abdominal venous pooling.9
Dietary changes can significantly improve the quality of life of POTS patients. Gluten-free diets in particular have shown improvement in autonomic symptoms, most notably orthostatic intolerance, vasomotor symptoms, and gastrointestinal symptoms.62 Smaller portioned meals may decrease postprandial gut venous pooling, and high-fiber diets can improve GI symptoms such as constipation.9
Finally, for acute decompensation and for patients who cannot tolerate oral hydration due to GI symptoms, intravenous saline infusion may alleviate symptoms, although some studies have demonstrated saline’s efficacy in symptom improvement for non-acute presentations.5,63 Additional non-pharmacologic therapies focus on symptomatic improvement, such as breathing physiotherapy.6
Pharmacologic Therapies
Currently, there are no Food and Drug Administration (FDA)-approved treatments for POTS, and pharmacologic agents used in POTS patients are considered off-label.8 Medication decision-making should incorporate specific patient presentations and observed associations, as well as an informed discussion between the patient and prescriber about the risks and benefits.
Medications That Target Vascular Tone
Midodrine, an α1-adrenergic agonist, is an effective treatment for POTS secondary to abnormal vascular tone by increasing vasoconstriction and, consequently, improving venous return to the heart.64 Evening doses of midodrine should be avoided due to supine hypertension.9 Octreotide is another vasoconstricting medication that primarily targets the splanchnic vasculature; however, octreotide is administered as a subcutaneous or intramuscular injection, which may limit adherence.65 Droxidopa is a synthetic amino acid precursor of norepinephrine and assists in increasing vasoconstriction peripherally with minimal effects on blood pressure.5,8 Finally, stimulant medications such as modafinil, in addition to improving symptoms of brain fog and fatigue, can induce peripheral vasoconstriction, although these medications may also increase blood pressure and tachycardia.5,65
Medications That Target Rapid Heart Rate
β-blockers are frequently used for tachycardia in POTS patients. These medications reduce cardiac baroreceptor activation, lower serum norepinephrine levels, and inhibit sympathetic nerve activity.66 Although the non-selective β-blocker propranolol is most prescribed, a randomized clinical trial by Moon et al. found that both propranolol and bisoprolol, a β1-adrenergic receptor-selective antagonist, were efficacious in improving quality of life and depressive symptoms for POTS patients.67 Broadening the scope of β-blockers to longer-acting β-blockers serves a potential role in treatment. Calcium channel blockers may be considered in patients who cannot tolerate β-blockers, although they carry the risk of worsened venous pooling. Verapamil, in particular, may be helpful in patients with a ‘hyperadrenergic’ subtype and may alleviate migraine symptoms.7
Ivabradine, a drug approved by the FDA for symptomatic heart failure with reduced ejection fraction, is being increasingly used in POTS patients due to its cardiac selectivity.68 Ivabradine blocks the funny (If) current of the sinoatrial node, thereby lowering heart rate and increasing diastolic time without lowering blood pressure.68 This medication may be particularly useful for the hyperadrenergic form of POTS. Taub et al. performed a clinical trial that demonstrated ivabradine’s efficacy in improving both heart rate and quality of life in hyperadrenergic POTS patients.69 Ruzieh et al. found that numerous POTS symptoms, including palpitations, lightheadedness, syncope, fatigue, brain fog, and shortness of breath, improved after ivabradine use.70
Pyridostigmine acts to inhibit acetylcholinesterase, increasing the availability of acetylcholine at ganglionic nicotinic receptors and postganglionic muscarinic receptors. This increases parasympathetic and cardiovagal tone, resulting in reduced heart rate.6 Kanjwal et al. found that pyridostigmine use improved fatigue, palpitations, presyncope, and syncope due to improvements in hemodynamics without reflex tachycardia.71 Notably, GI upset is a frequently reported side effect of this therapy.
Medications That Target Hypovolemia
In addition to salt supplementation, fludrocortisone, a glucocorticoid that acts similarly to aldosterone, has shown efficacy in improving POTS symptoms.65 However, patient adherence due to weight gain may be a challenge, as well as the need for routine monitoring of serum potassium levels for hypokalemia.65 Desmopressin is a vasopressin analog that similarly increases blood volume, although desmopressin has fewer vasopressor effects than vasopressin.72 Coffin et al. showed that desmopressin administration significantly decreased standing heart rate without significantly changing blood pressure.72 Erythropoietin can increase vascular volume but requires frequent complete blood count checks and increases the risk of venous thromboembolism, stroke, and MI.11,65 In a retrospective analysis, Kanjwal et al. found clinical improvement in patients using erythropoietin whose symptoms were refractory to other medications.73
Medications That Target Sympathetic Activity
Clonidine, an α2-adrenergic receptor agonist, improves POTS symptoms by decreasing sympathetic activity and reducing autonomic instability.9 Methyldopa, a longer acting α2-adrenergic receptor agonist, is reported to be better tolerated by POTS patients than clonidine.74 These medications may be beneficial and tolerated better in hyperadrenergic POTS than in neuropathic POTS.8
Medications with Undefined Mechanisms or Subtype-specific Targets
Selective serotonin reuptake inhibitors (SSRIs) have demonstrated symptomatic improvement in POTS patients.75 These medications increase monoamine neurotransmitter availability in the synaptic cleft to increase neurotransmission.75 SSRIs can also improve vasovagal syncope through an unknown mechanism, although peripheral vasoconstriction through increased norepinephrine bioavailability may play a role.75 Mar et al. found that sertraline may have a mild pressor effect, but no significant effect on heart rate or symptom improvement.75 In addition, atomoxetine, a serotonin–norepinephrine reuptake inhibitor (SNRI), has been studied in POTS patients and has demonstrated worsening of self-reported symptoms and increases in standing and sitting heart rate.76 Atomoxetine is occasionally prescribed by physicians in an attempt to increase peripheral vasoconstriction, but may lead to a worsening of symptoms.76 Bupropion has shown improvement in syncope, although no effect on heart rate, and, in our clinic, has assisted patients who present with brain fog.77
Therapies directed towards mast cell activation may also provide benefit in patients with POTS, particularly those with suspected coexisting MCAS. First-line treatment for MCAS is avoidance of or desensitization to triggers.78 In addition, beneficial pharmacologic therapies include histamine antagonists (at both H1 and H2 receptors), mast cell stabilizers such as cromolyn sodium, and leukotriene receptor antagonists such as montelukast.18 Molderings et al. have published a comprehensive review of MCAS therapies, although POTS-specific management related to MCAS has not been thoroughly explored.78
For patients with autoimmune-associated POTS, particularly those diagnosed with SFN, IVIG and plasmapheresis have demonstrated improvement in symptoms.79 Kesterson et al. explored less invasive subcutaneous administration of immunoglobulin in patients with various autoimmune associations and severe symptomatic POTS and found that subcutaneous immunoglobulin administration was beneficial in alleviating patient-reported symptoms.32
Interventional Therapies
Although the use of implantable devices and procedural interventions are rare in POTS patients, a subset of patients may respond well to interventional therapies. Patients who experience neurocardiogenic syncope as part of their POTS presentation should be evaluated for the use of cardiac implanted electronic devices.12 Loop recorders may be used to identify arrhythmia causes of neurocardiogenic syncope, such as asystole or bradycardia.6 These patients may see symptomatic improvement with dual-chamber pacemaker placement. Kanjwal et al. demonstrated the elimination of syncope after pacemaker implantation in 40 patients with asystole on loop recording, although symptoms of tachycardia and dizziness may persist.80
POTS patients who experience supraventricular tachycardia identified by an implantable loop recorder may respond well to cardiac ablation. Atrioventricular node ablation is an infrequently reported intervention that may alleviate symptoms in patients refractory to medical therapy.81 Sinus node ablation is not recommended due to increased risk of procedural complications, such as phrenic nerve paralysis and escalation of interventional therapy to a permanent pacemaker.82 de Asmundis et al. explored a sinus node-sparing technique using video-assisted epicardial ablation with endocardial 3D mapping that resulted in normal sinus rhythm in 11 POTS patients who underwent the procedure.82 Results from sinus node-sparing ablation therapy appear more efficacious and safer than radiofrequency sinus node ablation.83
Areas of Interest for Further Research
Although the therapies described above have been commented on in the POTS literature, there are other therapies that are worth mentioning that have not been formally studied but have anecdotally helped patients with POTS and would be interesting areas of future research. Many young women with POTS find exacerbation of symptoms around the time of their menstrual cycle or ovulation, possibly due, in part, to renin–angiotensin–aldosterone system dysregulation.84,85 Regulating the hormonal cycle with continuous oral contraceptive therapy or placement of an intrauterine device with the guidance of a gynecologist can greatly improve the quality of life for these patients. Not only does the hormonal regulation help relieve excessive menstrual bleeding, limiting anemia and iron deficiency, but it also removes a potential trigger of symptoms by minimizing fluctuations in estrogen levels.84
Oral or IV iron supplementation in patients with or without anemia also provides subjective symptom relief. As we know from the heart failure literature, supplemental IV iron, but not oral iron, improves patient-reported symptoms and quality of life and may have beneficial effects for preventing hospitalization and mortality.86 Another area of further exploration is the treatment of venous insufficiency or pelvic venous congestion, which would limit venous pooling and help with hypovolemic POTS symptoms.49
Conclusion
POTS is a heterogeneous disorder with variable clinical presentations and a substantial impact on patient quality of life. Associations with multiple disorders have been identified and may provide insight into previously unrecognized etiologies, novel pathophysiology, and optimal management of patient symptoms. Therapies vary widely for POTS and successful treatment may be viewed as an art focused on symptom improvement and the treatment of comorbid or underlying conditions rather than a standardized algorithm. Improved characterization of POTS symptoms and subtypes will be beneficial in designing ideal treatment plans for this population.