Patients with acute MI routinely undergo coronary angiography to identify obstructive coronary artery disease (CAD). However, MI and non-obstructive coronary arteries (MINOCA) accounts for up to 5–15% of patients presenting with acute coronary syndrome (ACS) and is associated with poor cardiovascular outcomes despite the lack of epicardial obstructive atherosclerotic plaque.1–3 The American Heart Association Scientific Statement defines MINOCA as meeting the definition of an acute MI, having absence of ≥50% obstruction in any major epicardial vessel on coronary angiography, and no other clinically overt non-ischemic diagnoses, such as sepsis, pulmonary embolism, takotsubo, and myocarditis.1 An acute MI is defined as an increase and/or decrease in cardiac biomarkers (with at least one value being above the 99th percentile of the upper reference limit) coupled with clinical evidence of infarction (either through ischemic changes on EKG, clinical symptoms, imaging evidence of myocardial damage such as wall motion abnormality, and/or intracoronary thrombus).4 MINOCA is a working diagnosis that should prompt further investigation beyond routine coronary angiography because identifying a more specific mechanism affects choice and duration of therapy tailored for that individual.5 The purpose of this review is to highlight the pathophysiology, epidemiology, diagnosis, treatment, and prognosis of MINOCA as well as to identify knowledge gaps in the management of this condition.3,6,7
Definitions and Pathophysiology
MINOCA can occur with either angiographically normal coronary arteries or mild-to-moderate atherosclerosis (<50% stenosis). It is important to distinguish that MINOCA by definition means that MI has occurred, whereas non-obstructive CAD with evidence of myocardial ischemia without infarction is increasingly being referred to as ischemia and no obstructive coronary arteries (INOCA).8,9 INOCA is diagnosed in stable patients without current evidence of infarction but is also associated with major adverse cardiovascular events (MACE), including recurrent MI and hospitalization for angina.10 While a patient with a history of INOCA can have a MINOCA event, the degree of overlap between INOCA and MINOCA is not entirely clear.
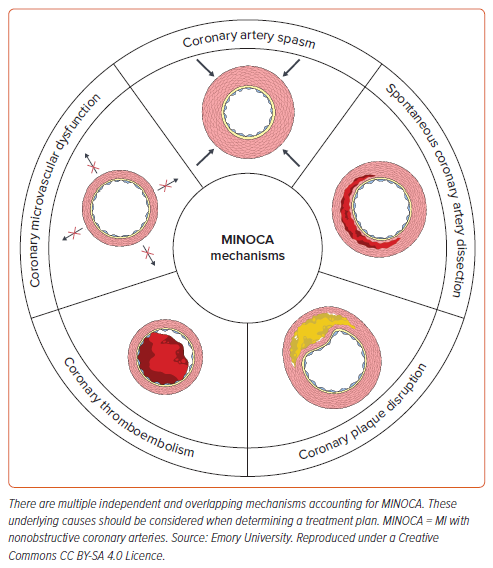
In a patient who presents with a MINOCA event, there are five main coronary causes that should be considered as an etiology:
- Coronary plaque disruption
- Coronary artery spasm (CAS)
- Spontaneous coronary artery dissection (SCAD)
- Coronary microvascular dysfunction (CMD)
- Coronary thromboembolism (Figure 1).
However, the cause of MINOCA in an individual patient may be multifactorial. For example, CAS and CMD may occur together to cause a MINOCA event, and both should be considered during management.
The most common mechanism of MINOCA appears to be atherothrombosis related to fibrous cap plaque disruption. Although there is no obstructive disease, diffuse atherosclerosis with positive remodeling is often present.11,12 Studies have demonstrated that 38% of MINOCA patients have plaque disruption on intravascular ultrasound (IVUS).13,14 Plaque disruption is a broad term that includes both plaque rupture and plaque erosion. Atherosclerotic plaques that are more prone to rupture are characterized by a large lipid core covered with a thin fibrous cap. Plaque rupture triggers clot formation and leads to an occlusive red thrombus leading to angina and MI. However, fibrous plaques usually have a small lipid-rich core and are more likely to undergo erosion rather than rupture. They are associated with non-occlusive white thrombus caused by erosion of the endothelial layer.15 Apoptosis of the endothelial lining leads to loss of barrier function and enables thrombus formation on the eroded endothelium. The thrombus can undergo fibrinolysis or distal embolization which may limit detection on angiography.13,16
In the HARP study, Reynolds et al. reported that atherothrombotic mechanism accounted for a vast majority of MINOCA. Using multi-modality imaging with three-vessel optical coherence tomography (OCT) at the time of index coronary angiogram, and combining this information with cardiac MRI (CMR), they were able to identify the underlying mechanisms in 85% of patients with MINOCA.17 Comprehensive coronary function testing to test vascular function abnormalities was not done in this study and therefore, CAS and CMD as other contributing mechanisms were not evaluated. Zeng et al. found that of 190 MINOCA patients who underwent OCT, 52.1% had an atherosclerotic cause for MINOCA – plaque erosion in 33.7%, plaque rupture in 17.4%, and calcified nodule in 1.1%. Non-atherosclerotic causes included SCAD (4.2%) and CAS (4.2%), while 38.9% were unclassified. Of note, CAS was identified based on OCT findings and invasive provocation testing was not performed in this study.18
While the definition of vasospasm varies in the literature, CAS is typically defined as >90% vasoconstriction of an epicardial coronary artery.1,19 Studies based on provocative vasospasm testing, usually performed by administering intracoronary acetylcholine, found that 10–46% of patients with MINOCA had inducible CAS.3,20,21 One proposed mechanism for predisposition to CAS is nitric oxide synthase deficiency and the resulting decrease in vasodilatory nitric oxide, which leads to endothelial dysfunction and altered vascular tone that favors vasoconstriction.22 Similarly, vascular smooth muscle cells may have intrinsic hyperreactivity secondary to hereditary changes in pathways that regulate vasoconstriction.23
While epicardial CAS may lead to episodic vasospastic angina, it should be noted that coronary microvascular vasospasm can also lead to intermittent angina at low workloads and with emotional stress. During invasive coronary function testing, microvascular vasospasm is diagnosed when the patient experiences symptoms and has ischemic EKG changes without any visible epicardial vasospasm on angiography in response to acetylcholine.24,25 CAS is estimated to account for 20% of MINOCA cases. Triggers for CAS include smoking, hyperventilation, exposure to cold, acute mental stress, and use of recreational drugs, such as cocaine and marijuana.6,26–28
CMD is another mechanism that may contribute to MINOCA.29 In CMD, there are functional and/or structural alterations in the microcirculatory resistant vessels (those <500 µm in diameter) that lead to impaired coronary flow reserve.30–33 Chronic exposure over time to cardiovascular risk factors such as hypertension, diabetes, and inflammation lead to endothelial dysfunction, promote arteriolar remodeling, and induce microvascular damage, leading to microvascular ischemia. CMD is usually diagnosed using PET-derived myocardial flow reserve or using invasive coronary function testing. Comprehensive coronary function testing can identify both CMD and CAS.
Approximately 10% of MINOCA events occur due to coronary thromboembolism. It is important to rule out intracardiac sources including paradoxical embolism from right-to-left shunt lesions, such as patent foramen ovale, apical thrombi, valvular heart disease, and infective endocarditis.34 Individuals with thrombophilia disorders, such as Factor V Leiden, protein C and S deficiencies, may be at higher risk for MINOCA due to the elevated risk of spontaneous thrombosis. In one meta-analysis that included an analysis of the prevalence of inherited thrombotic disorders in patients with MINOCA across eight studies, 14% of the 378 patients with MINOCA tested positive for an inherited thrombotic disorder on thrombophilia screening tests. These clots may be undetectable on angiography either due to their size or rapid fibrinolysis.3
Finally, SCAD is an important non-atherosclerotic mechanism of MINOCA and may be easily missed without careful review of the invasive coronary angiogram or coronary CT angiography.35 In this condition, which predominates in women, a spontaneous tear in the arterial wall leads to a cascade of events, including hematoma formation that can propagate distally and compress the true arterial lumen.36 In particular, type II SCAD is characterized by long, diffuse, and smooth artery narrowing, which may be misinterpreted as a non-obstructive small caliber vessel. SCAD is associated with arteriopathies, such as fibromuscular dysplasia; connective tissue disorders, such as Marfan’s or Ehlers-Danlos; hormone fluctuations; and stressful events.37,38
One must keep in mind that not all troponin elevation is due to myocardial ischemia.39 Common non-coronary causes that lead to troponin elevations are myocarditis and cardiomyopathy, and these conditions should be considered as an explanation in the working diagnosis of MINOCA. In addition, other causes that lead to troponin elevation, such as tachyarrhythmias, pulmonary emboli, sepsis, renal disease, and anemia should be considered.
Epidemiology and Risk Factors
The prevalence of MINOCA in large national studies is reported to be between 5–15% of individuals presenting with acute MI. The clinical characteristics of patients with MINOCA often differ from the characteristics of patients with MI from obstructive CAD (MI-CAD). In a systematic review of patients with MINOCA, Pasupathy et al. noted that patients with MINOCA are often younger (mean age 59) and are more likely to be women when compared to patients with MI-CAD (mean age 61).3 Furthermore, in the VIRGO study, one in eight women with acute MI had MINOCA, and it was more prevalent in patients <55 years.6
In the Acute Coronary Treatment and Intervention Outcomes Network Registry, a large multicenter registry of 322,523 patients who presented with MI, MINOCA was present in 10.5% of women versus 3.4% of men.2 Additionally, those with MINOCA are less likely to have traditional cardiovascular risk factors, such as hyperlipidemia, than those with MI-CAD.40 However, recent studies suggest that non-traditional risk factors such as depression, stress, hypercoagulable states, and autoimmune disease are more prevalent in the MINOCA population compared to the MI-CAD population.6,41
Psychological stressors are especially important factors associated with heart disease in women and are prevalent in women regardless of the type of MI. A recent study of 486 women with acute MI (172 women with MINOCA versus 314 with MI-CAD) measured perceived stress and depressive symptoms at the time of MI and 2 months post-MI. High stress levels were reported by 51% of patients with MINOCA versus 63% of patients with MI-CAD (p=0.021), and depressive symptoms were reported by 36% of patients with MINOCA versus 43% of patients with MI-CAD (p=0.229). There were no differences in depressive symptoms at the time of MI or at 2 months post-MI among the two MI groups.42
Diagnosis
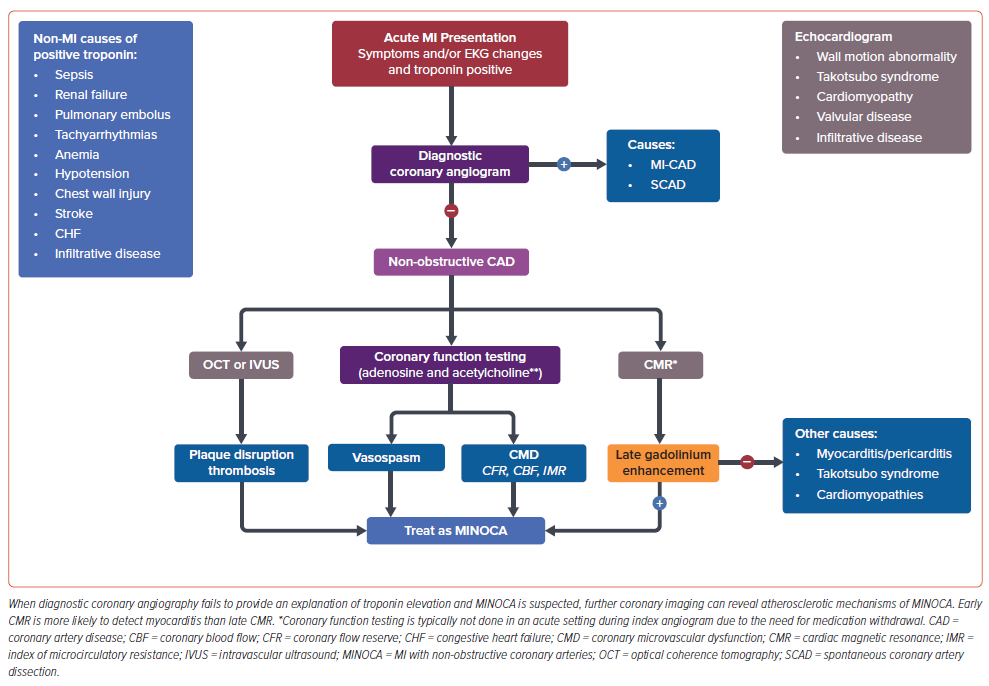
Despite differences in demographic characteristics, there are no differences in symptoms that can help differentiate MINOCA versus MI-CAD and thus all patients should be treated initially for ACS. On EKG, the majority of patients with MINOCA lack ST elevations but 20–30% of patients with MINOCA present as ST-elevation MI (STEMI).2,3,40,43,44 Once a patient has been determined to have an acute MI via biomarkers and corroborative clinical evidence, an angiogram is usually performed to determine if there is an obstructive lesion (>50% stenosis or hemodynamically significant culprit lesion). 1,45 During the initial angiogram, images should be reviewed carefully to ensure that SCAD or coronary thromboembolism have not been missed. If there is no obstructive CAD or obvious cause of troponin elevation identified, then MINOCA is diagnosed. At this point further diagnostic coronary imaging, such as IVUS or OCT can be considered to clarify atherosclerotic mechanisms of MINOCA, such as plaque erosion, rupture, or calcified nodule (Figure 2).5
In the management of MI, transthoracic echocardiogram (TTE) is usually performed to assess ventricular function and wall motion abnormalities.1 For the work-up of MINOCA, TTE may identify important alternative diagnoses including cardiomyopathy or takotsubo syndrome; however, when the cause of MINOCA is not clear, CMR is recommended since it can aid in the diagnosis of myocarditis, takotsubo syndrome, and other cardiomyopathies.46,47 Notably, CMR findings in both myocarditis and myocardial infarct may include systolic dysfunction, T2 weighted enhancement with edema, and late gadolinium enhancement, so the full clinical context is important to consider.46,48 Of note, CMR cannot always detect small areas of myocardial injury, and therefore, a small MI could have occurred even if CMR is normal. Furthermore, since the timing of CMR is important to rule out myocarditis, it is best to obtain CMR imaging during the index hospitalization.
In the SMNC-2 study, CMR with advanced tissue characterization performed at a median of 3 days compared to 12 days after hospitalization was more likely to provide a diagnosis of myocarditis and takotsubo syndrome.49 A prior meta-analysis showed that 33% of patients suspected of MINOCA actually had myocarditis on CMR and only 24% had typical findings of a subendocardial infarct.1,50,51 The European Society of Cardiology (ESC) guidelines for patients presenting with ACS without persistent ST elevations recommend CMR in all patients with MINOCA who do not have an obvious underlying cause (Class 1B).52
If these modalities have yet to confirm a diagnosis and the patient is having recurrent and persistent chest pain after their MINOCA event, coronary functional assessment to diagnose coronary endothelial dysfunction, CMD, or CAS can be considered. Typically, this invasive coronary function testing is not done at the time of index MI hospitalization, partly due to logistical reasons – a comprehensive coronary function test requires that vasoactive medications such as β-blockers, calcium channel blockers (CCB), and nitrates are withheld 24 to 48 hours prior to testing to allow accurate physiologic measurements. Furthermore, the choice of pursuing additional invasive testing may also depend on patient preference, recurrent symptoms, test availability, local expertise, and cost considerations. The test itself assesses endothelium-dependent (acetylcholine response) and endothelium-independent (adenosine response) vascular function. CMD is diagnosed when there is either an impaired coronary flow reserve (CFR) of less than 2.5 with no obstructive CAD or an abnormal index of microcirculatory resistance (IMR≥25).5,25 Abnormal vasoconstriction in response to intracoronary acetylcholine is used to diagnose coronary endothelial dysfunction and/or CAS.19
Treatment
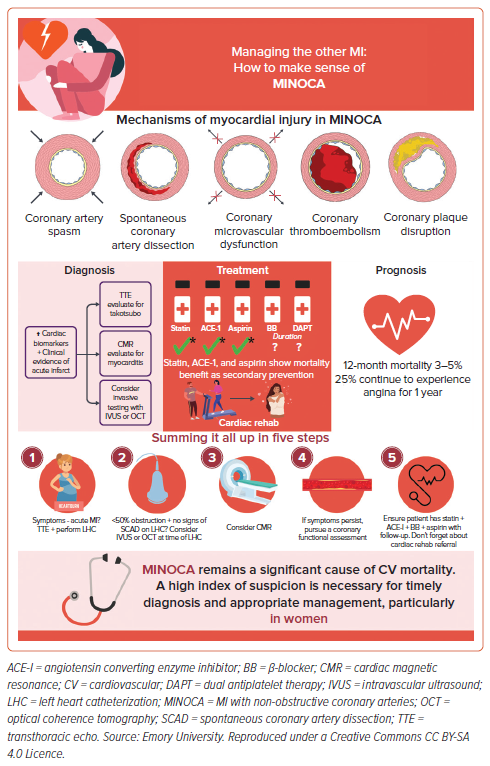
The duration of therapy and optimal medical treatment is not well-established for patients with MINOCA with no recurrent angina.1 At initial presentation, the management of MINOCA is similar to the management of patients with MI-CAD. Once angiography demonstrates that there is no obstructive CAD, treatment should be tailored to the underlying pathophysiologic mechanism of MINOCA. The SWEDEHEART study showed a significant mortality benefit in patients with MINOCA on statins and angiotensin converting enzyme inhibitors (ACE-I) [HR 0.77 [0.68–0.87] and HR 0.82 [0.73–0.93], respectively] for secondary prevention.7 A Korean registry also showed that prescription of renin-angiotensin system blockers and statins at discharge was associated with lower mortality in patients with MINOCA.1,53 It is thus recommended that all patients with MINOCA should receive statin, ACE-I, β-blocker, and aspirin (Figure 3). Additionally, patients should be referred for cardiac rehabilitation which can confer significant benefits post MI.
Mechanism-specific management of MINOCA, however, is important. Patients with plaque disruption that leads to MINOCA share a similar mechanism to patients with MI-CAD and should thus be treated with the same mainstays of medical management listed above including dual antiplatelet therapy (DAPT). Patients in whom CAS is suspected as the cause of symptoms may benefit from CCB and nitrates. An observational study of 327 SCAD patients showed both benefits of using β-blockers and lower risk of SCAD recurrence.54
Patients with coronary artery thrombosis or emboli should be managed on an individual basis with some requiring thrombectomy while others with risk factors predisposing to thrombus, such as AF, needing anticoagulation. Those with CMD may require a combination of β-blockers, CCB, ranolazine, ACE-I, nitrates, and statins. In patients with CMD, if there is persistent angina despite usual anti-anginal therapy, other therapies that target nociception such as tricyclic antidepressants, enhanced external counterpulsation, neuromodulatory strategies such as spinal cord stimulator, cognitive behavioral therapy, and a coronary sinus reducer device can be considered.9 The PROMISE trial is under way and is randomizing MINOCA patients to specific treatment groups to better identify optimal therapeutic strategies.55,56
The duration of DAPT for MINOCA patients is not clear. The ESC guidelines advocate 1 year followed by indefinite single antiplatelet therapy in cases where plaque disruption is the suspected mechanism.5 The American Heart Association (AHA) recommends aspirin monotherapy with consideration of a second antiplatelet agent.1 However, these strategies are extrapolated from data on acute MI-CAD management. The primary study referenced in both guidelines is the SWEDEHEART trial, which did not show a statistically significant treatment benefit of DAPT at 1 year for patients with MINOCA; however, this conclusion was limited by the fact that patients were not divided by MINOCA etiology, but were in a single cohort including all etiologies.7
Several clinical research trials have focused on improving outcomes for patients with MINOCA. The PROMISE trial aims to assess if there is improvement in quality of life, prognosis, and healthcare costs by tailoring medical therapy based on underlying pathogenesis of MINOCA. Secondary objectives include exploring the diagnostic roles of microRNAs in MINOCA and evaluating if CMR can aid in risk stratification for these patients.55 Additionally, the MINOCA-BAT trial involved randomizing patients to different treatment arms and investigating cardiovascular endpoints to determine optimal therapeutic strategy.56 β-blockers are used for MINOCA if the underlying mechanism is either SCAD due to the established decreased risk of recurrence or CMD as antianginal therapy.54 However, long-term use of β-blockers has not been shown to be of clear benefit in patients with history of MI who do not have heart failure or left ventricular systolic dysfunction.
The SWEDEHEART trial failed to show a statistically significant treatment benefit of β-blockers at 1-year in patients with MINOCA.7 The WARRIOR trial focuses on individuals with non-obstructive CAD, aiming to determine whether intensive medical therapy, including high-intensity statins and ACE-I/ARB, improves MACE compared to standard care. Although this population differs from those with MINOCA, the findings from this study may have implications for the long-term treatment of non-obstructive CAD in patients with MINOCA.57
In a registry-based study of 5,913 patients with MINOCA admitted to high-volume hospitals between 2007 to 2014, Smilowitz et al. found considerable variability in discharge prescriptions of ACE-I/ARB and ß-blockers. A median of 45.6% (interquartile range [IQR]: 38%–56.5%) patients received a prescription for ACE-I/ARB, while a median of 74.1% (IQR: 64.7%–80%) were discharged on β-blockers. Of note, patients with MINOCA who had another indication for ACE-I/ARB or β-blockers, such as cardiomyopathy with ejection fraction (EF) 40%, diabetes, or chronic kidney disease were excluded.58
Outcomes and Prognosis
Patients with MINOCA are at higher risk of cardiovascular mortality and morbidity compared to the general population.40 While MACE rates are lower compared to patients with MI-CAD, MINOCA is not benign. MINOCA has been associated with in-hospital mortality of 3–5% at 1 year and 24% risk of MACE, defined in this study as a composite of cardiac mortality, reinfarction, heart failure and stroke at 4 years.40 The VIRGO study showed 1-year mortality in patients with MINOCA to be approximately 5%, though 1-year mortality was between 1–2% in patients younger than 55 years.6 Outcomes and prognosis are likely to be dependent on the mechanism of MINOCA, although few studies have evaluated this.3,7,40 Additionally, Zeng et al. reported that the 1-year composite MACE rate – including death, MI, stroke, target lesion revascularization, and angina rehospitalization – was 15.3% for atherosclerotic causes versus 4.5% for non-atherosclerotic causes of MINOCA.18
While some studies suggest MINOCA has a long-term favorable prognosis compared to MI-CAD, other studies suggest these two types of MI may have similar short- and long-term prognosis.40,59 In a study of MI in young patients under the age of 45 years, those with MINOCA had lower cardiovascular mortality than those with MI-CAD, yet comparable rates of reinfarction, ischemic stroke, and all-cause mortality after a 20-year follow up.59 Approximately 25% of patients with MINOCA also continue to experience angina for the first 12 months after their acute event.60 Those with MINOCA who present with STEMI have been noted to have a higher 40% 5-year all-cause mortality rate despite having fewer cardiovascular risk factors than those with STEMI in the setting of coronary obstruction.6,40,61 The outcomes of patients with MINOCA and MI-CAD were also compared in a south-east Asian cohort of predominantly women.43 While the patients with MINOCA had a lower incidence of MACE, heart failure hospitalization, and all-cause mortality compared to those with MI-CAD, an alarming prognosis was noted in this population, with 1 in 5 patients with MINOCA experiencing MACE. Predictors of adverse cardiovascular outcomes included STEMI at presentation, older age, lower renal function, and absence of antiplatelet use.
Observational studies in those with MINOCA have identified factors that increase risk of MACE. Older age, hypertension, smoking, reduced ejection fraction, chronic obstructive pulmonary disease, and elevated creatinine were found to predict MACE after adjustment in a cohort of over 2,000 patients.62 In addition to the risk factors above, independent predictors of all-cause death included cancer and elevated C-reactive protein in this population. Lower total cholesterol was shown to be a protective factor for MACE in patients with MINOCA and patients with MI-CAD, indicating a potential benefit of targeted statin therapy.62
There may also be a significant impact on resources incurred from MINOCA. Studies showed that patients with MINOCA have higher readmission rates than patients with MI-CAD.63 The diagnostic work up of MINOCA does require further investigation that can incur costs and therefore may also lead to longer hospitalizations. However, identifying MINOCA is important given that these patients are at higher risk of future events. Indeed, studies have shown that MI is more likely to be missed in women due to non-classic presentations, such as shortness of breath, dizziness, nausea, or unusual fatigue.44 An early recognition of MINOCA during the work-up may therefore ensure early appropriate treatment. A subset of patients with MINOCA have recurrent chest pain without MI, and patients with INOCA have been shown to have recurrent hospitalization, repeat testing, high use of healthcare resources, and poor quality of life.64
Knowledge Gaps
More studies are needed to further understand gender and ethnic differences in MINOCA. It remains unclear why young women are more susceptible to MINOCA, but they may have unique psychosocial risk, stress, hormonal factors, and inflammation that contribute. Prior work has shown that myocardial ischemia induced by mental stress is more prevalent in young women.28 It is unclear why some patients with MINOCA have persistent angina, while others have relatively well controlled symptoms or are asymptomatic. It is possible that those with recurrent chest pain post-MINOCA have coronary vascular dysfunction (either CMD and/or CAS) but acquired nociceptive abnormality could also be a contributor. Both an improved pathophysiological understanding and risk stratification tools are needed to identify the subgroup of stable INOCA patients who may be at risk of a future MINOCA event. Also needed are randomized trials testing whether comprehensive coronary function testing (using both adenosine and acetylcholine) plus intracoronary imaging with optical coherence tomography (OCT) or IVUS during the index hospitalization for acute MI improves outcomes in MINOCA. The benefit of DAPT beyond 1 year in MINOCA management is unknown, and more clinical trials testing the efficacy of other therapeutics across all subtypes of MINOCA are needed.
Conclusion
MINOCA is increasingly being recognized as an important contributor to adverse cardiovascular outcomes, particularly in young women. It can present as both STEMI and non-STEMI, and multiple pathophysiologic mechanisms can trigger MINOCA. Identification of the underlying cause of a case of MINOCA has therapeutic implications. If there is no obstructive CAD on coronary angiography, further catheter-based coronary imaging with IVUS or OCT can be helpful to clarify the cause since MINOCA is considered a working diagnosis. CMR plays an important role in ruling out myocarditis and cardiomyopathies. Secondary prevention with ACE-I and statins can be used in MINOCA, but the indications for DAPT and other therapies in MINOCA management beyond 1 year is unknown. Treatment trials to guide care and improve outcomes in this population are urgently needed.