Cardiac surgery results in profound physiological changes in cardiac function that are mediated by changes in cellular metabolism related to cardioplegia, direct mechanical myocardial and pericardial injury, and the systemic inflammatory response caused by tissue damage and cardiopulmonary bypass.1–4 The postoperative cardiac surgery population is at a high risk of developing any combination of shock phenotypes (cardiogenic, obstructive, hemorrhagic, and vasoplegic shock), which require rapid identification and intervention. This review highlights considerations unique to the diagnosis and treatment of post-cardiotomy shock.
Stepwise Assessment
In the immediate post-surgical period, shock is common and often multifactorial in etiology. In practice, managing post-cardiotomy shock typically requires differentiating between and determining the severity of frequent contributions to shock following cardiac surgery, including postoperative vasoplegia, bleeding, pump failure, and tamponade.
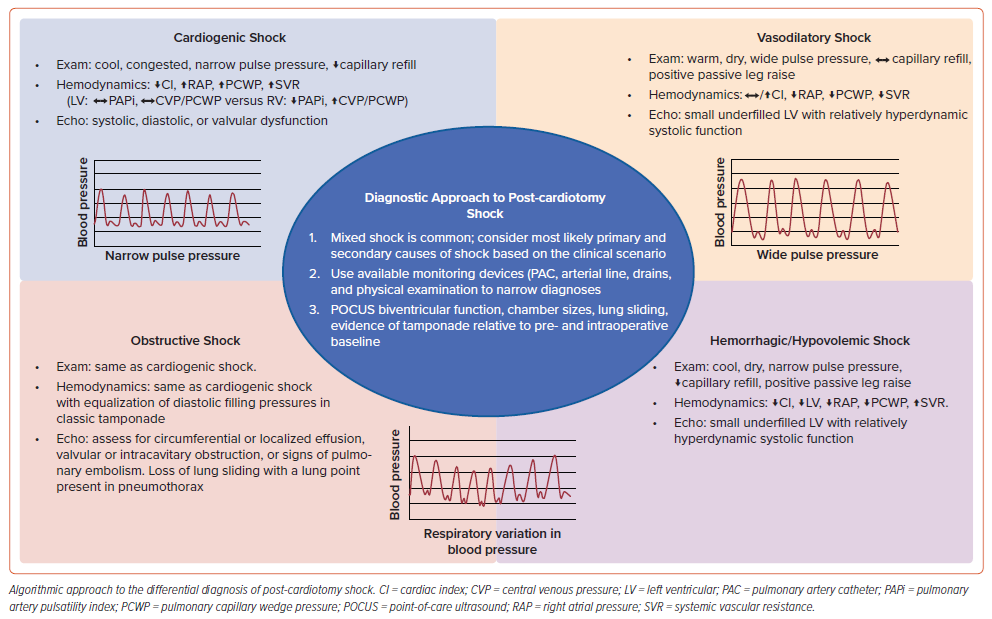
We take a stepwise approach that uses bedside assessment, invasive hemodynamics, point-of-care ultrasound (POCUS), and ancillary data to determine the predominant drivers of post-cardiotomy shock in any given case (Figure 1). Our strategy is to first assess the degree of vasoplegia and volume responsiveness by incorporating the central venous pressure (CVP), pulse pressure, pulse pressure variation, and response to a passive straight leg raise.5 Second, we evaluate for bleeding by assessing chest tube/drain output, hemoglobin trends, and using POCUS of the chest to rule out hemothorax. Third, using invasive hemodynamics, we evaluate biventricular function by assessing both the left and right ventricular filling pressures, pulsatility index, and cardiac index. Lastly, we use POCUS to assess biventricular size and function, rule out significant valvular disease, and rule out the presence of a pericardial effusion, with transesophageal echocardiography (TEE) used when transthoracic POCUS views are inadequate.6 Where there is diagnostic uncertainty, invasive hemodynamics combined with TEE can be particularly helpful.
The above strategy is only one such approach to shock following cardiac surgery, with the most important point being that having a systematic approach, testing hypotheses, and not anchoring on any one diagnosis is often best practice.
Vasodilatory Shock: Diagnosis
Vasodilatory shock is characterized by profound vasodilation and reduced systemic vascular resistance (SVR).7 Vasoplegia is common following cardiac surgery, occurring in 20% of patients, and is strongly associated with poor outcomes.8,9
Vasoplegia is thought to result from multiple factors but primarily reflects a systemic inflammatory response induced by cardiopulmonary bypass.8 Risk factors for post-bypass vasoplegia include duration of cardiopulmonary bypass, preoperative angiotensin-converting enzyme (ACE) inhibitor use, preoperative vasopressor use, and preoperative renal dysfunction.9,10 The European Association for Cardio-Thoracic Surgery guidelines assign a class IC recommendation to discontinue ACE inhibitors and angiotensin II receptor blockers preoperatively.11
Clinical evidence suggestive of vasodilatory shock includes low SVR, preserved or increased cardiac output relative to baseline, warm extremities, pulse pressure variation with respiration, and wide pulse pressure (assuming the patient does not have severe aortic insufficiency, severe peripheral arterial disease or an arteriovenous fistula).8,12 Vasodilatory shock is often accompanied by relative hypovolemia as well, and thus these patients may have low filling pressures and a positive response to a straight leg raise.
Alternative etiologies of vasodilatory shock include those common to all critically ill patients, including sepsis, adrenal insufficiency, anaphylaxis, pancreatitis, and liver failure.7,8 Major infection following cardiac surgery is uncommon (3.5% within 30 days following coronary artery bypass grafting [CABG]) although it is associated with significant mortality.13 Early sepsis following cardiac surgery can be difficult to diagnose, particularly in the immediate postoperative period, as postoperative vasoplegia and sepsis both present with vasodilatory shock.14 Inflammatory markers are typically elevated following cardiac surgery and cardiopulmonary bypass, although procalcitonin has shown some efficacy in distinguishing post-cardiotomy septic shock.14 Blood cultures may be negative in the immediate postoperative period due to routine intraoperative antibiotic use.14
Two specific populations at particularly high risk of postoperative sepsis are patients undergoing surgery for infectious endocarditis and patients undergoing orthotopic heart transplant (OHT). Surgery for endocarditis can release endotoxins, resulting in immediate postoperative septic shock, most commonly due to staphylococcal endocarditis.15 Among patients who undergo OHT, infection is the second most-common cause of mortality within 30 days of surgery.16 Because they need immunosuppression, post-transplant patients are vulnerable to a wide variety of pathogens. Early infections (<30 days postoperatively) are predominantly bacterial (with a slight preponderance of Gram-negative over Gram-positive organisms), although viral infections, especially cytomegalovirus, are also common.16,17 Fungal infections, primarily Aspergillus and Candida, can also be seen following OHT.16,17
Days to weeks after surgery, infections can arise from sternal infection, mediastinitis, and vein graft harvest sites, as well as other sources of infection common to all critically-ill patients such as pneumonia, catheter-associated infection, and urinary infections.13,14 Mediastinitis, which is associated with bilateral internal mammary grafts, obesity, chronic obstructive pulmonary disease, and diabetes, is an especially morbid infection.18 Prevention of surgical site infection is a major focus of cardiac surgery practice, and typical strategies include perioperative antimicrobial administration, perioperative skin decontamination with antiseptic, and meticulous surgical technique.
Finally, protamine, a fish-derived protein commonly used for reversal of heparin anticoagulation after cardiac surgery, has been associated with multiple rare adverse reactions, including transient mild hypotension, anaphylaxis, and severe pulmonary vasoconstriction leading to right ventricular (RV) failure.19 The risk is highest in patients who have been previously exposed to protamine, including in neutral protamine Hagedorn insulin. These reactions typically occur shortly after administration but have been reported up to 20 minutes after exposure.
Vasodilatory Shock: Treatment
There is relatively scant trial data to guide management of vasodilatory shock following cardiac surgery. If volume-responsive hypotension is likely, often based on relatively low CVP, augmented pulse pressure variation, and a positive response to a straight leg raise, then a fluid bolus can be trialed first to address a relative hypovolemic component of shock. A positive response to fluid bolus should demonstrate an improvement in blood pressure, reduction in vasopressor dose, increased cardiac output and/or greater urine output. At times, several liters of fluid over the initial resuscitation period will be needed to maintain an adequate intravascular volume. Reassessment before each volume administration is necessary as excess fluid administration can be harmful.
There has been a significant amount of interest in albumin as a resuscitation fluid in cardiac surgery. While the specific type of fluid administered is a matter of ongoing debate, we generally use crystalloid, as a randomized controlled trial showed no difference in outcomes between lactated Ringer’s solution and albumin when administered intraoperatively during cardiac surgery.20 A recent large meta-analysis did not find a benefit from albumin in patients undergoing cardiac surgery.21 However, institution-specific guidelines for post-cardiotomy fluid administration may still show a preference for colloid resuscitation, particularly for the stage D heart failure population after heart transplantation or durable left ventricular assist device (LVAD) implantation.
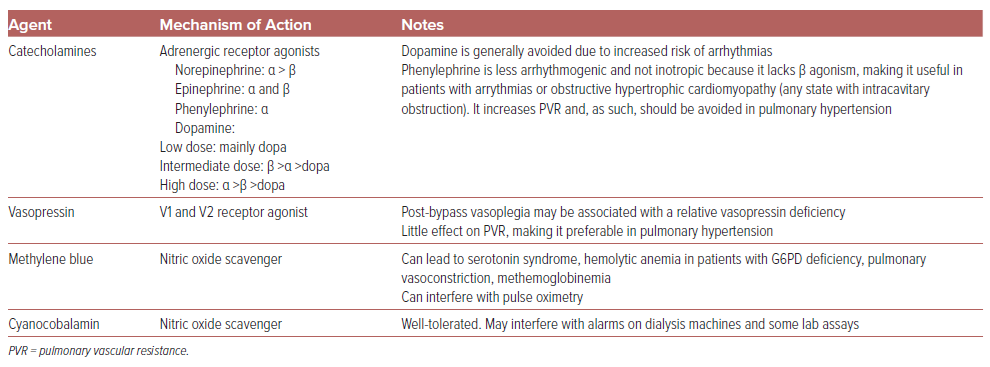
In addition to fluid administration, vasopressor use is common, with catecholamines being the most frequently used vasopressors following cardiac surgery (Table 1).22 Norepinephrine is the most commonly used catecholamine in the postoperative setting, followed by phenylephrine and epinephrine.22 Dopamine (dosed up to 20 μg/kg/min) has been proved to result in a greater number of arrhythmias than norepinephrine (dosed up to 0.2 g/kg/min) in one small randomized clinical trial of patients with post-cardiotomy shock, so is used infrequently in this population.23
Vasopressin is also an important vasoconstrictor as post-bypass vasoplegia may be associated with relative vasopressin deficiency.24 The single-center randomized VANCS trial showed a significant reduction in the composite endpoint of stroke, prolonged mechanical ventilation, sternal wound infection, reoperation, or renal failure with vasopressin compared to norepinephrine, along with a lower incidence of atrial fibrillation.25
Other agents may also be helpful for refractory vasoplegic shock, including methylene blue (which carries multiple potential side effects) and cyanocobalamin.26,27 Glucocorticoids have been generally found to shorten the duration of vasodilatory shock due to sepsis, but minimal data exist regarding their utility in non-septic forms of vasoplegic shock including post-cardiotomy shock.8,27 Optimal management of postoperative vasoplegia remains an area of significant uncertainty and ongoing research, particularly regarding novel vasoactive agents. A randomized trial comparing prophylactic methylene blue and hydroxycobalamin in cardiac surgery (NCT03446599) was anticipated, but could not be completed due to lack of funding, leaving non-randomized data as the primary source of evidence at this point regarding these medications.
Post-cardiotomy Cardiogenic Shock
There is varying nomenclature for cardiogenic shock (CS), including cardiac insufficiency and low cardiac output syndrome, but CS is typically defined as hypoperfusion caused by a cardiac etiology, and is generally characterized by reduced cardiac output (typically a cardiac index ≤2.2 l/min/m2).28
Clinical factors that suggest cardiogenic shock include low cardiac output, high SVR, cool extremities, delayed fingernail capillary refill time, narrow pulse pressure, elevated filling pressures (which may not always be present), and low urine output.29,30 A normal left ventricular ejection fraction does not rule out a cardiogenic etiology of shock, while a low left ventricular ejection fraction (particularly when stable from baseline) does not rule out alternative mechanisms of shock.5 Blood pressure can be variable in patients with post-cardiotomy cardiogenic shock (PCCS).5
In patients in whom invasive hemodynamics are not available, POCUS assessment of left ventricular outflow tract (LVOT) velocity time integral can provide a rapid approximation of stroke volume, while mitral E/e’ ratio can help with classifying patients at the extremes of intracardiac filling pressures.5
The reported incidence of PCCS varies with patient and surgical factors, ranging from 2.4% following isolated CABG to 3.9% after aortic valve surgery and 7% following mitral valve surgery.31–33 PCCS is associated with significant morbidity and nearly 20% mortality.31–33 Risk factors for PCCS include older age, female sex, preoperative renal failure, preoperative CS, and preexisting left ventricular (LV) dysfunction.31,32 The commonly used Society of Thoracic Surgery (STS) and European System for Cardiac Operative Risk Evaluation (EuroSCORE-2) scores do not directly predict the frequency of PCCS.34 PCCS can develop intraoperatively, resulting in difficulty separating from cardiopulmonary bypass, or postoperatively.35 The Society for Coronary Angiography and Intervention (SCAI) shock classification system, whereby patients are classified across the spectrum of shock severity from A to E (with A representing patients at risk and E patients in extremis), has been shown to be a useful risk-stratification tool in PCCS.36
PCCS frequently results from myocardial dysfunction of the left ventricle, the right ventricle, or both. While often the pathophysiology is primarily a cardiomyopathic process (pump failure), it should be kept in mind that PCCS can be secondary to other cardiovascular etiologies, including myocardial stunning, myocardial ischemia, valvular/structural abnormalities, intracavitary dynamic obstruction, dysrhythmias, and restrictive physiology. This section will focus on the management of pump failure, and discuss considerations for the diagnosis and management of secondary causes of myocardial dysfunction in the post-cardiotomy patient.
Left Ventricular Failure: Diagnosis and Management
LV systolic failure is the most common cause of PCCS.37 Secondary causes of LV dysfunction (such as ischemia) should be assessed while acting quickly to achieve the twin goals of maintaining organ perfusion and promoting myocardial recovery. Goal-directed therapy to achieve specific hemodynamic targets has been associated with reduced complications in high-risk cardiac surgery patients.38
Approximately 30% of patients undergoing cardiac surgery receive inotropic medications postoperatively.22 Patients with isolated hypotension without hypoperfusion (SCAI stage B) PCCS have a good prognosis and should generally be managed pharmacologically.36,39 For patients with hypoperfusion or who fail to respond to inotropes, temporary mechanical circulatory support (tMCS) should be strongly considered. It should be noted that late deterioration (worsening hemodynamics after the initial 24 hour postoperative period) carries a worse prognosis than early PCCS, even with SCAI stage B PCCS, and vigilance should be maintained to ensure adequate pharmacological and/or mechanical support.36
Mortality in CS is much higher once multi-organ failure develops, and it is recommended that support is escalated before the onset of organ injury or significant lactate elevation (i.e. >4 mmol/l).40,41 An additional non-pharmacological intervention to consider for PCCS is mechanically increasing the heart rate via pacing to augment cardiac output, particularly in the setting of low stroke volume inadequately responsive to inotropes.
Due to surgically related cardiac ischemic time, cardioplegia, and myopericardial inflammation, patients will universally have some degree of diastolic dysfunction following cardiac surgery.2 Isolated diastolic dysfunction is rarely the primary driver of cardiogenic shock, but in conjunction with other processes such as arrhythmias (especially atrial fibrillation) or unrepaired structural heart disease can lead to shock.37 Overall principles for the management of diastolic dysfunction include optimizing cardiac filling pressures, maintaining atrioventricular synchrony, and avoiding excessive tachycardia.42
Right Ventricular Failure: Diagnosis and Management
Post-cardiotomy RV failure is associated with elevated mortality and prolonged length of stay, with an incidence that varies widely across studies.43,44 Risk factors for post-cardiotomy RV failure include LVAD implantation, OHT, and preexisting pulmonary hypertension.30,44
Broadly, RV failure can occur due to intrinsic RV dysfunction, high pulmonary vascular resistance, or volume overload.30 The risk of RV failure following durable LVAD placement is particularly high given the acute increase in cardiac output to which the RV is not accustomed.45 Air embolism to the right coronary artery, given its superior location when the patient is supine intraoperatively, is also an important cause of perioperative RV failure.46
Causes of RV failure which are common to all critically ill patients can afflict post-cardiotomy patients as well, and include acute respiratory distress syndrome, pulmonary embolism, and high intrathoracic pressures.37
Making a diagnosis of RV failure can be difficult, in part because there is no standardized definition. One recent definition of postoperative RV failure uses a combination of low RV stroke work index (<4, where RV stroke volume index = [0.136 × stroke volume × (mean pulmonary artery pressure minus right atrial pressure)]/body surface area), absence of elevated left-sided filling pressures, and either high CVP (≥15 mmHg) or low cardiac index (≤1.8 l/min/m2).30 The CVP/pulmonary capillary wedge pressure ratio (with a threshold of ≥0.63 after LVAD implantation or ≥0.85 after acute MI) and pulmonary artery pulsatility index are also used as markers of RV function.47 The pulmonary artery pulsatility index is calculated as ([pulmonary artery systolic pressure − pulmonary artery diastolic pressure]/CVP), with a threshold of ≤1.0 for acute MI-CS patients after percutaneous coronary intervention or ≤1.85 for LVAD patients to define RV failure.47,48
The presence of CVP in both of these metrics highlight its importance in the diagnosis of RV failure, while serial measurements of pulmonary artery pulsatility index have been shown to more accurately predict RV failure compared to a single measurement.49 Echocardiographic assessment of the RV is complex, with reduced tricuspid annular plane systolic excursion (abnormal <18 mm), lateral tricuspid annular excursion velocity (abnormal <10 cm/s), and fractional area change (abnormal <35%) among the most widely adopted metrics, although these are less easily acquired technically and less specific in the post-cardiac surgery population.50
The general tenets for the management of RV failure in the post-cardiotomy period are volume optimization, afterload reduction, and inotropy and/or tMCS. There is nuance to each of these tenets and each case must be navigated by serial clinical assessment.
In terms of preload, the RV filling pressure needed to optimize Frank-Starling forces may initially be on the higher side due to the post-surgical reduction in myocardial compliance. Given that the RV is particularly sensitive to elevated afterload, minimizing hypoxia, correcting acidosis, and avoiding excessively high or excessively low lung volumes are generally recommended clinical practices in all patients.51,52,53
Pharmacological afterload reduction to a specific target, though, requires special consideration and an understanding of the patient’s cardiovascular physiology. If the driver of elevated afterload is the pulmonary vasculature (precapillary), then a pulmonary vasodilator such as inhalation nitric oxide or inhalation epoprostenol may provide benefit.54,55 However, if the driver of increased afterload is elevated left-sided filling pressures or mitral pathology (postcapillary), then pulmonary vasodilators can cause pulmonary edema and worsen gas exchange by increasing flow to the noncompliant pulmonary venous circulation or left heart. In such situations, the goal is to unload the left ventricle by targeting lower mean arterial pressure, preload optimization, and inotropy or left-sided tMCS when appropriate. Additionally, given the anterior location of the RV, it is particularly vulnerable to compression in the setting of myocardial and/or chest wall edema, so delayed sternal closure can be a useful strategy in some patients with RV failure.56
Among vasoactive agents, we recommend against the use of phenylephrine for RV failure because it has been shown to increase pulmonary vascular resistance and reduce cardiac output, whereas vasopressin, norepinephrine, epinephrine, and milrinone may have neutral to beneficial effects on pulmonary vascular resistance and right ventricular–pulmonary artery coupling, making them generally preferred in RV failure.53
Recognition of perioperative RV failure is often delayed, and early mechanical circulatory support (MCS) deployment should be considered in severe RV failure given its association with improved outcomes.57–59 Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is generally the preferred tMCS device when RV failure is accompanied by LV failure, with an RV assist device alone or with an oxygenator (creating a veno-venous ECMO circuit) used for isolated RV failure or RV failure with respiratory dysfunction, respectively, although biventricular tMCS configurations are also possible.34,41 Management of patients with preexisting RV failure carries increased complexity, and is well described elsewhere.53 The use of vasoactive agents in RV failure following cardiac surgery will also be informed by a forthcoming trial (NCT04501861) comparing hemodynamic changes and RV function between norepinephrine and vasopressin.
Secondary Causes of Post-cardiotomy Cardiogenic Shock: Diagnosis and Management
Ischemic injury is an important contributor to PCCS. The reported incidence of MI following cardiac surgery ranges from <1 to ~10%.60 Diagnosing an MI following cardiac surgery (termed a type 5 MI when following CABG) can be challenging given patients invariably have elevated cardiac biomarkers and frequently have EKG changes due to epicardial injury or a ventricular paced rhythm.61 The fourth universal definition of MI defines a type 5 MI as an elevation of cardiac troponin of >10 × the 99% percentile of the upper reference limit (URL) following CABG accompanied by imaging or EKG evidence of ischemia.61 Recent data suggest that the threshold level of high-sensitivity troponin associated with mortality is actually much higher at 218 × the URL for CABG or aortic valve surgery and 499 × the URL for other cardiac surgery.62 Thus, overall, cardiac enzyme elevation is rarely the primary driver of clinical decision making unless extremely elevated, and clinical management is instead based on holistic patient evaluation. A creatine phosphokinase-MB test can be helpful, given its more rapid clearance compared to cardiac troponin, resulting in a greater temporal ability to detect new ischemic insults.60
Post-cardiotomy ischemia can result from a variety of factors, including graft-related factors following CABG, intraoperative ischemia-reperfusion injury, coronary artery injury, and an acute coronary lesion.60 Acute graft-related factors can include technical errors, thrombosis, and spasm. A recent American Heart Association scientific statement included an algorithm for management of postoperative ischemia following cardiac surgery.60 Patients who are hemodynamically stable should, after discussion with the primary cardiac surgeon, undergo coronary angiography with percutaneous coronary intervention of the native coronaries preferred when possible over graft intervention, which can be technically challenging. However, patients who have high suspicion for surgical bleeding or mechanical graft complications should undergo initial surgical re-exploration and revision. Vasodilators may be helpful in the setting of graft spasm.
There are multiple other secondary causes of post-cardiotomy cardiogenic shock. LVOT obstruction and systolic anterior motion of the mitral valve are rare but often underrecognized etiologies. Systolic anterior motion and LVOT obstruction occurring postoperatively are often associated with hypertrophic cardiomyopathy or mitral valve surgery (occurring in 4.6% of patients following mitral valve repair in one study).63,64 LVOT obstruction can also be seen following aortic valve replacement for aortic stenosis, stress cardiomyopathy, MI with hyperdynamic function of the LV basal wall segments, and in vasoplegic shock with a small, hypercontractile LV.65 Management of LVOT obstruction includes augmenting diastolic filling by increasing preload, decreasing inotropy, decreasing chronotropy, and increasing LV afterload.65 A common strategy includes volume expansion, short acting β-blockers (such as metoprolol tartrate or esmolol), and the use of non-β-adrenergic vasopressors (such as vasopressin or phenylephrine).
Atrial arrhythmias are common following cardiac surgery, most notably AF, with an estimated incidence of up to 60% after combined CABG plus valve surgery.66 A randomized controlled trial showed no difference in outcomes between rate versus rhythm control for AF after cardiac surgery, although patients could be transitioned from rate to rhythm control if achieving sinus rhythm was felt necessary to improve their hemodynamics.67
Loss of organized atrial activity can lead to hemodynamic decompensation, particularly in the presence of significant diastolic dysfunction (with or without concomitant systolic dysfunction), and thus electrical or pharmacological cardioversion of atrial arrhythmias should be considered in PCCS. Achieving euvolemia, transitioning vasopressors to non-β agonists (e.g. to vasopressin or phenylephrine) and addressing electrolyte and/or metabolic derangements may also help maintain sinus rhythm in this population. If it is difficult to determine a patient’s atrial rhythm using a surface EKG/telemetry, the atrial lead of epicardial pacing wires can be connected to an EKG machine to obtain an atrial intracardiac electrogram.68
Inotropic Agents and Pacing Strategies
Inotropic options include epinephrine, milrinone, and dobutamine (and, in some countries, levosimendan). Inotropes are generally the first-line option for PCCS, with the goal of improving cardiac output and markers of perfusion.
Milrinone and dobutamine are both inodilators, and thus are best used in patients with normal to elevated blood pressure. A small randomized trial in PCCS compared milrinone (starting dose 0.5 μg/kg/min) to dobutamine (starting dose 10 μg/kg/min), and showed a more rapid increase in cardiac index with dobutamine over the first 2 hours after initiating therapy, with no difference at 4 hours.69
Milrinone is renally cleared and has a longer half-life than dobutamine and, as such, dobutamine is generally preferred in patients with renal dysfunction or hypotension. Milrinone is a modest pulmonary vasodilator, leading to an equivalent decrease in pulmonary vascular resistance as the use of 20 ppm of inhaled nitric oxide.70 Epinephrine, an inopressor, is also used frequently in the post-cardiotomy population. Epinephrine is associated with increased lactate levels, which can make it challenging to distinguish tissue hypoperfusion from epinephrine-mediated lactate elevation.71 Little data currently exist to guide inotropic agent and dose selection for PCCS.
Dual chamber (atrial and ventricular) pacing is preferred when possible to maintain atrioventricular synchrony and the atrial contribution to ventricular filling. Biventricular pacing (typically epicardial) has also been shown to increase cardiac output and decrease the requirement for vasoactive medications.72 Overdrive pacing can be used to attempt to terminate reentrant arrhythmias.73 It should be noted, particularly in the case of ventricular overdrive pacing for ventricular tachycardia, that this can lead to rhythm degeneration into VF. Overdrive pacing can also be used to suppress ectopy and minimize the risk of an R-on-T phenomenon and resulting torsades de pointes in the setting of a prolonged QT interval.
Left-sided Temporary Mechanical Circulatory Support Devices
Approximately 50% of patients with PCCS will undergo tMCS placement, with the intra-aortic balloon pump (IABP) the most commonly used device.74 The IABP is typically placed percutaneously via the femoral artery and uses counterpulsation in the descending thoracic aorta to directly increase coronary perfusion and reduce LV afterload to indirectly augment cardiac output.75 IABP cardiac output augmentation is modest, usually in the range of 0.5 l/min, although some patients may have a more robust response.76
Preoperative tMCS is used in approximately 2% of all patients undergoing cardiac surgery, with the highest rates in patients undergoing CABG, with IABP being the most frequent tMCS device.77 Selective preoperative IABP use in high-risk patients is associated with improved outcomes and there are data suggesting the pulsatile flow of an IABP may help to minimize the negative effects of the non-pulsatile flow of cardiopulmonary bypass intraoperatively.79,79 On the other hand, placement of an IABP intraoperatively has been associated with increased mortality, and some authors do not feel that an IABP alone provides adequate support for most patients with post-cardiotomy CS.34,80 Despite this, a recent consensus statement describes IABPs as the ‘mainstay’ of CS management in this patient population due to its ease of use and safety profile.41
The TandemHeart system (LivaNova) is a left atrial (via transseptal puncture) to femoral or iliac artery bypass device powered by an external centrifugal pump that can provide up to 5 l/min of flow. There are minimal data describing TandemHeart use specifically for perioperative or PCCS hemodynamic support.81
The Impella platform (Abiomed) offers other tMCS devices that use a microaxial continuous flow pump to directly transfer blood from the left ventricle to the aorta.82 Early generations of the Impella device (5.0/LD), which are no longer in use, used direct surgical insertion into the ascending aorta and were studied in the setting of PCCS.83 The Impella CP (a 14 Fr device) can provide up to 4 l/min of flow and is typically placed percutaneously via the femoral artery. The larger Impella 5.5 (19 Fr) provides higher levels of flow (up to 5 l/min), but requires surgical implantation into the subclavian/axillary artery, which also allows for patient ambulation.
The Impella catheter is contraindicated in patients with LV thrombus, severe aortic regurgitation, or aortic dissection, and cannot be used in patients with a mechanical aortic valve. The Impella 5.5 is some clinicians’ preferred tMCS strategy for isolated post-cardiotomy LV failure given the potential for full or nearly full hemodynamic support with a configuration that allows for early mobilization and physical therapy participation.34 However, data supporting this practice are scant, including small randomized trials (not specific to the 5.5 device nor the post-cardiac surgery population), and observational studies (primarily in acute MI-CS using the Impella CP) that have generally observed improved hemodynamics but increased complications with an Impella device relative to an IABP.84
The recent DanGer Shock study, which randomized patients with acute MI-CS to coronary revascularization with or without Impella CP support, was the first clinical trial to show a mortality benefit for any tMCS device.85 Notably, only about 2% of the study participants underwent cardiac surgery with CABG. As this trial did not enroll patients with PCCS, it remains to be seen how this study may influence care patterns regarding early tMCS in PCCS or preoperatively in patients at risk for PCCS. The prospective, single-arm Impella-Protected Cardiac Surgery Trial of prophylactic Impella 5.5 placement in patients undergoing high-risk cardiac surgery should provide more insight into Impella use more specifically for the cardiac surgery population.86
Right-sided Temporary Mechanical Circulatory Support Devices
Temporary MCS devices that provide direct RV support transfer blood from the right atrium (RA) or central veins to the pulmonary artery, bypassing the RV.87 The ProtekDuo system (LivaNova) consists of a single large (29–31 Fr) dual lumen cannula, which is placed via the right internal jugular vein. Blood is withdrawn from ports in the right atrium and vena cava into an external pump and returned to the pulmonary artery, and the device can provide 4–5 l/min of blood flow. An oxygenator can be added to this system to allow for respiratory support via ECMO. The Impella RP Flex (Abiomed) is another primary form of percutaneous RV support, capable of providing 4 l/min of blood flow via a smaller (11 Fr) cannula. This catheter-based Impella device is placed via the internal jugular vein into the pulmonary artery and pumps blood from the RA directly to the pulmonary artery.
Adverse events with right-sided Impellas are generally similar to those occurring with other Impella devices, and include hemolysis and thrombosis. The risk of hemolysis may be decreased by ensuring that the Impella has an unobstructed inlet and that the outlet is approximately 4 cm above the pulmonic valve.88 The ProtekDuo and Impella RP flex are continuous-flow devices that bypass the RV, decreasing right-sided filling pressures while increasing pulmonary pressure and flow, making invasive and noninvasive metrics of RV function difficult to interpret.87
Biventricular Support
VA-ECMO provides cardiopulmonary support via biventricular bypass. A continuous flow pump withdraws venous blood, passes it through an oxygenator to allow gas exchange, and returns the blood to the arterial system.89
A VA-ECMO device can be placed peripherally (generally through femoral arterial and venous cannulation) or centrally into the great vessels or heart itself, which typically requires the chest to remain open postoperatively. In patients who are unable to be weaned off bypass, central VA-ECMO can be initiated intraoperatively using the same cannulation sites as used for bypass.90 In the post-cardiotomy CS population, peripheral VA-ECMO is the most common configuration.91
Each cannulation strategy carries its own trade-offs. Peripheral VA-ECMO is less invasive, carries a lower risk of infection, and has a lower bleeding risk. Peripheral VA-ECMO from a femoral approach delivers blood to the aorta in a retrograde fashion, significantly increasing LV afterload (potentially necessitating a second MCS device or surgical LV vent to unload the LV) and can lead to differential hypoxia between the upper and lower portions of the body (North-South or Harlequin’s syndrome).92 There is also a risk of leg ischemia with femoral arterial cannulation, which is significantly reduced by placing a distal perfusion catheter to deliver antegrade flow to the leg.89 Alternatively, central ECMO delivers blood to the proximal aorta antegrade, minimizing increases in LV afterload and differential hypoxemia, while also achieving higher flows than peripheral cannulation.92 However, central cannulation is associated with higher mortality in a propensity-scored model from the Extracorporeal Life Support Organization (ELSO) registry.91
There are a variety of other possible cannulation strategies to provide LV, RV, or biventricular support.93 Beyond tMCS, durable LVADs can also be used as an initial strategy for patients with CS, although there are minimal data regarding this rare approach.94
Survival following PCCS managed with VA-ECMO is low, with reported in-hospital survival of 42% in the ELSO registry, 40% in the international PELS-1 study, and 36% in the PC-ECMO registry.91,95,96 Survival in this population is comparable to that of patients managed with VA-ECMO for acute MI-CS.97
Older age is a strong risk factor for in-hospital mortality in patients with PCCS managed with VA-ECMO in multiple cohorts, with survival of 39% for patients aged <60 years versus 13% aged ≥80 years in the PC-ECMO study.91,95,96 Female sex is also a risk factor for mortality.98 In the PELS-1 cohort, a large proportion of in-hospital deaths occurred while on VA-ECMO support (37% mortality at a median of 79 hours on ECMO), while survivors and patients who died after ECMO weaning both subsequently had prolonged intensive care unit (ICU) stays of approximately 3 weeks.99 Given these considerations, discussions of goals of care should be initiated and involve palliative care consultants early in this patient population.
Obstructive Shock
Etiologies of obstructive shock include cardiac tamponade, pulmonary embolism, tension pneumothorax, and auto-positive end expiratory pressure in mechanically ventilated patients. This review will focus on tamponade as it is the primary concern in post-cardiotomy patients.
Cardiac tamponade occurs when pericardial pressure exceeds intracardiac pressure, resulting in cardiac chamber collapse, impaired cardiac filling, and subsequent low cardiac output leading to shock.100 In contrast to the classic presentation of cardiac tamponade with circumferential pericardial effusions with predictable intracardiac hemodynamics, tamponade in the post-cardiotomy patient may result from localized pericardial and or mediastinal effusions causing collapse of only a single cardiac chamber.
One study of patients with surgically confirmed tamponade on re-exploration characterized the differences between patients who presented with early (<72 hours following cardiac surgery) versus late tamponade.101 Among patients with early tamponade, 92% had localized pericardial effusions, which were not detected by transthoracic echocardiography (TTE) in 60% of cases. Only 21% of these patients had classic echocardiographic findings of tamponade. In contrast, patients who presented with late tamponade were much more likely to have large, circumferential effusions that were detected by TTE with characteristic findings of tamponade physiology.
In patients who are hypovolemic, tamponade physiology can occur with low filling pressures, and should be initially treated with fluid resuscitation.101 Definitive management of cardiac tamponade consists of emergent drainage of the effusion, which can be done percutaneously if technically feasible, but typically requires surgical re-exploration for localized effusions.100 Aggressive fluid administration to increase intracardiac filing pressures and vasoactive medications can be used as temporizing measures until drainage is achieved.
Intubation (in patients who are not already intubated) and high-pressure ventilation should be avoided if possible as tamponade hemodynamics depend on high sympathetic tone and venous return to maintain preload. In patients where tamponade is suspected but not seen by TTE, TEE should be strongly considered given the greater sensitivity for localized effusions.101
Hypovolemic/Hemorrhagic Shock
Cardiopulmonary bypass has multiple effects on the coagulation system that predispose to bleeding, including hemodilution, consumption of coagulation factors, residual heparin effect, and hypothermia.102 Blood transfusions are common, with 40–80% of patients receiving blood products during the intra- or postoperative phase.103
Peri-operative hemorrhage is classified according to the universal definition of perioperative bleeding in cardiac surgery, where severe bleeding is defined as requiring >5 units pf packed red blood cells or fresh frozen plasma, re-exploration or delayed sternal closure, or >1 l of postoperative chest tube blood loss within 12 hours.104
In one large study of CABG patients, risk factors associated with the need for reoperation for bleeding included emergency surgery, preoperative renal dysfunction (especially dialysis), male sex, and cardiogenic shock, with reoperation required in 2.4% of patients.105 A nationwide analysis from the UK’s National Health Service found that the incidence of post-cardiac surgery bleeding complications was 6.7%, with surgical re-exploration needed in 0.3%, and that bleeding was strongly associated with greater mortality (OR 3.44), length of stay, and costs.106 Aspirin is generally continued through surgery, while P2Y12 inhibitors and oral anticoagulants are generally discontinued several days before surgery given the strong association with bleeding.11
Hemorrhagic shock should be suspected in any post-surgical patient in shock, particularly with significant intraoperative blood loss or significant chest tube output postoperatively. Retroperitoneal bleed, typically following femoral instrumentation, although potentially spontaneous, is a subtle source of bleeding which should be considered as well.
Kirklin and Blackstone indications for re-operation for bleeding are standard; the criteria include cardiac tamponade, a sudden increase in chest tube output (≥300 ml/h), marked early widening of the cardiac silhouette, or very high chest tube output (including >500 ml in the first hour or 1,200 ml in the first 5 hours).107 Very low chest tube output is also concerning, as it may indicate a clotted tube resulting in intrathoracic accumulation of blood predisposing to cardiac tamponade.
Hemorrhagic shock is classically associated with low intracardiac filling pressures, although mixed shock is not uncommon in the post-cardiotomy population, and normal-to-elevated filling pressures alone do not rule out a hemorrhagic component of shock.
There are two main categories of postoperative bleeding: failure of hemostasis due to coagulopathy without a specific source of bleeding (medical bleeding) and failure of hemostasis due to surgical causes with a specific bleeding source, often accompanied by mild coagulopathy (surgical bleeding).102,108
Guidelines recommend the use of viscoelastic testing (rotational thromboelastography or thromboelastography to allow for targeted hemostatic management within the OR, and these tests are also commonly used to address postoperative medical bleeding as well.109 Details of viscoelastic testing are beyond this scope of this manuscript but are well summarized elsewhere.102,109
Transfusion carries risks, and the TRICS III randomized clinical trial demonstrated the non-inferiority of a restrictive transfusion threshold hemoglobin of <7.5 g/dl in cardiac surgery patients.110 For patients with severe medical bleeding, prothrombin complex concentrate, tranexamic acid and/or recombinant factor VIIa can be used, although these agents carry a risk of thrombosis which must be considered.109
For patients with true hemorrhagic shock, surgical re-exploration should be strongly considered, as a large proportion of these patients will be found to have surgical bleeding requiring intervention to achieve hemostasis.102,108 In patients with significant hemorrhage resulting in instability, a massive transfusion protocol using a balanced 1:1:1 ratio of red blood cells, platelets, and plasma should be used.102 Additionally, proactive repletion of calcium is required during massive transfusion as calcium chelators are incorporated into the blood products.
Additionally, hypovolemia without hemorrhage is not uncommon in the post-cardiotomy patient. It is often related to increased capillary permeability leading to an efflux of plasma into the extravascular compartments (third spacing) along with a negative fluid balance from diuretics. The Fluids After Bypass trial tested a fluid administration protocol guided by cardiac output and stroke volume variation (with >13% indicating anticipated fluid responsiveness); patients in the protocol-driven arm received less fluid than the usual care arm but did not have a difference in the primary outcome of ICU length of stay.111
Cardiac Arrest in the Post-cardiotomy Patient
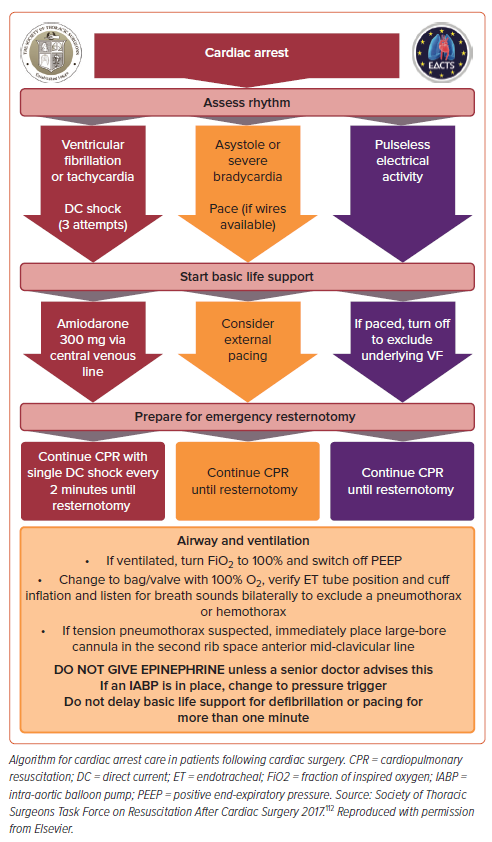
Cardiac arrest in the early post-cardiotomy patient is a different entity from arrest in the medical patient, and thus the standard medical Advanced Cardiovascular Life Support algorithm does not apply. Management of cardiac arrest is described in detail in a 2017 STS consensus statement (Figure 2).112 Crucially, survival in up to 50% of post-cardiotomy cardiac arrests can be seen, which compares favorably to 24% survival for in-hospital cardiac arrest in general.112,113 Cardiac arrest occurs in up to 8% of patients following cardiac surgery, with approximately 25–50% being due to shockable rhythms.112 Patients who arrest following cardiac surgery may experience myocardial damage with external cardiac massage (ECM; i.e. chest compressions), and cardiac tamponade is a significant and intervenable cause of postoperative arrest.
For these reasons, the STS guidelines emphasize rapid resternotomy within 5 minutes of arrest for patients within 10 days of cardiac surgery in whom return of spontaneous circulation is not immediately achieved with defibrillation or pacing, as indicated. For patients with shockable rhythms, three attempts at defibrillation (before ECM) should occur within 1 minute, followed by ECM while preparing for bedside sternotomy. For patients with asystole or severe bradycardia, pacing (either with internal pacing wires if present or external pacing) should be attempted for 1 minute before moving to resternotomy, with ECM in the interim. In patients with pulmonary endarterectomy, clinicians should proceed immediately to resternotomy.
The primary goal of emergent resternotomy is to relieve pressure on the heart caused by blood, fluid and alternative etiologies of obstructive shock. Internal cardiac massage carries risk of cardiac injury as well, and the guidelines state that “inexperienced providers should not rush to perform internal cardiac massage after opening the chest.”112 Before performing internal cardiac massage, the clot should be removed and all grafts should be identified. A two-handed technique is preferred for non-surgeons as it is felt to be the safest method.
Emergent resternotomy may be necessary in up to 2.7% of all post-cardiotomy patients and, given the time-critical nature of emergent resternotomy, non-surgeons, including advanced practice providers and senior ICU nurses, should be trained to perform resternotomy. For patients who undergo minimally invasive cardiac surgery without a sternotomy, external cardiac massage should be performed until an experienced surgeon is available to perform a sternotomy.
There are several other differences regarding the management of post-cardiac surgery arrest. The STS guidelines state that epinephrine should not be given routinely due to the risk of hypertension and bleeding in post-cardiac surgery patients who achieve a return of spontaneous circulation, but that lower doses of epinephrine of 50–300 μg may be useful in patients in extremis to prevent the development of arrest.112 In addition, mechanical CPR devices are assigned a class III recommendation for harm due to the risk of traumatic injury.
Outcomes Following Post-cardiotomy Shock
Overall, in-hospital mortality for patients following cardiac surgery is 2.6%.114 The risk of mortality rises significantly with an increase in the number of complications, ranging from 0.9 to 62.3% in one recent analysis that examined combinations of prolonged ventilation, stroke, reoperation, and renal failure.114 This has led to the development of the concept of failure to rescue as a quality metric of postoperative care, based on a hospital’s ability to prevent mortality among patients with postoperative complications.114 Importantly, cardiogenic shock is not included in this metric.
In-hospital mortality with PCCS varies by severity of shock, from 0.6% for SCAI stage B shock to 30.2% for SCAI E shock.36 The mortality for PCCS is lower than for CS unrelated to cardiac surgery for each SCAI stage.39 Limited data exist regarding long-term outcomes of survivors of post-cardiotomy shock, with most of the data being about patients with PCCS who were treated with VA-ECMO. In the PELS-1 cohort of PCCS patients treated with VA-ECMO, of those who survived to hospital discharge, 89.5% were still alive at 1 year.95
Conclusion
Patients who have undergone cardiac surgery are a distinct population with altered physiology and distinct pathophysiology compared to medical cardiology patients. Mixed etiologies of shock are common following cardiac surgery, and a stepwise approach to the diagnosis of shock is important to avoid anchoring bias, missed diagnoses, and failure to rescue. Preoperative optimization, including potential preoperative tMCS placement, should be considered. Ultimately, understanding the physiology and potential complications surrounding cardiac surgery will benefit clinicians and patients alike.