The US has the highest maternal mortality rate in the developed world, at an estimated 26.4 deaths per 100,000 live births. This rate is rising, although it is falling in other wealthy nations.1 Cardiovascular disease is a leading cause of maternal death, so cardiologists need to build on their knowledge and enhance their proficiency on the management of cardiovascular disease during pregnancy.2
There are numerous contributors of this rising risk, including advancing maternal age, pre-existing cardiovascular risk factors, the rise in multifetal pregnancies and survival to fertility age among childhood cancer survivors and women with congenital heart disease. Unlike most cardiovascular conditions, there are no large randomized controlled trials to guide decision-making, and guidelines are based principally on expert consensus.
It is increasingly likely that a cardiologist will be called upon to manage these women, so it is incumbent upon them to understand the basic cardiovascular hemodynamics of pregnancy and fundamental risk stratification and management of these conditions.
Hemodynamics of Pregnancy
The normal cardiovascular hemodynamic adaptations to pregnancy are remarkable but tolerated without difficulty in the majority of women. In women with cardiovascular dysfunction, however, these adaptations may precipitate cardiovascular decompensation.
Hemodynamic changes begin in the first trimester, with a 30–50 % rise in cardiac output, driven by an increase in stroke volume and, to a lesser extent, heart rate. Systemic vascular resistance falls as a result of endogenous vasodilators, as well as flow into the low-resistance uteroplacental unit. Red blood cell mass increases because of a rise in erythropoietin levels, though to a lesser extent than plasma volume, causing a dilutional anemia. Heart rate increases in the first trimester and reaches a peak in the early third trimester. Systemic blood pressure falls, reaches a nadir in the second trimester, then slowly increases to term.3 Notably, the supine position can cause a 24 % reduction in cardiac output, due to compression of the enlarging uterus on the inferior vena cava.4 This position can also increase afterload because of aortic compression. This is particularly important to consider during maternal cardiac arrest, at which time manual left lateral uterine displacement should be performed if the uterus can be palpated at or above the umbilicus.5
During labor and delivery, cardiac output is further increased because of auto transfusion from the contracting uterus as well as an increase in heart rate because of maternal pain during labor. Epidural anesthesia can produce transient hypotension resulting from acute venous and arterial dilatation, but then leads to improved hemodynamic stability because of attenuation of the effects on blood pressure and heart rate responses. Despite delivery, the postpartum phase remains an active period for the cardiovascular system and this is often when maternal decompensation occurs. There is further auto transfusion from uterine involution and mobilization of dependent edema. Because of the loss of the low vascular resistance placental unit, afterload increases. These adaptations are more marked in multifetal pregnancies.3
The normal echocardiogram in pregnancy reflects cardiac remodeling secondary to these physiologic changes. Left ventricular mass increases with eccentric hypertrophy and dilatation of all chambers can occur, although absolute values should remain in the normal range. Velocities across the valves increase and physiologic valvular regurgitation is noted in the tricuspid, pulmonary and mitral valves, but not the aortic valve at term. A small pericardial effusion can be seen in normal pregnancy. Diastolic function, as measured by load-independent indices, such as tissue Doppler imaging, are unchanged during pregnancy despite the increase in preload and physiologic hypertrophy.6–9
Risk Stratification
When considering pregnancy in a woman with heart disease, the following factors must be considered: maternal risks of morbidity and mortality; fetal risks of preterm birth, growth restriction or death; risk of congenital heart disease in the baby; and the risk of pregnancy on long-term maternal outcome.3
Patient counseling regarding the risks of pregnancy should ideally take place before conception. This allows informed decision-making about pregnancy risk and the optimization of maternal status, including careful review of potentially teratogenic medications and the use of alternatives. Several risk estimation models exist, notably the CARPREG, Zahara and WHO classification schemes.10–12 The original CARPREG risk score includes four variables: prior cardiac event; New York Heart Association (NYHA) functional classification status; impaired systemic ventricular function; and left-sided obstruction. The Zahara model is based exclusively on women with congenital heart disease and includes eight factors: history of arrhythmia; cardiac medications prior to pregnancy; NYHA status; moderate/severe systemic atrioventricular (AV) valve regurgitation; moderate/severe pulmonary AV valve regurgitation; left heart obstruction; the presence of a mechanical valve; and cyanotic heart disease. The third model, the WHO risk classification, defines specific conditions within a category of 1 through to 4, where women in category 4 are advised against pregnancy. A comparison of these three risk estimation methods demonstrated that all three models were predictors of maternal cardiac risk with the WHO classification having the best discriminatory capabilities.13
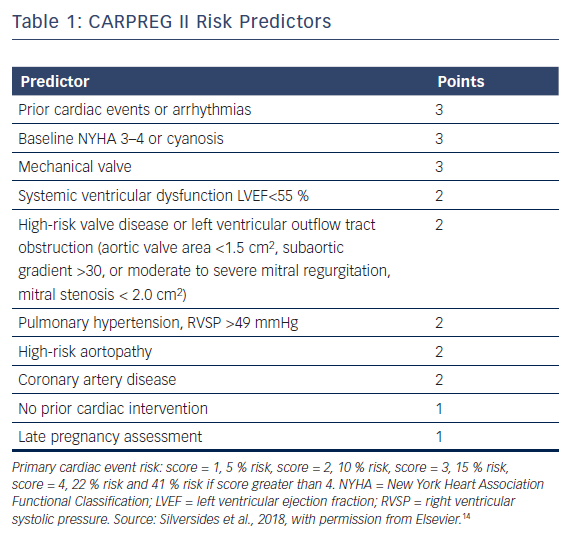
More recently, the CARPREG investigators derived an additional comprehensive risk stratification scheme named CARPREG II (Table 1).14 The predicted risk of primary cardiac event was 5 % with a score of 1, 10 % for a score of 2, 15 % with a score of 3, 22 % for a score of 4 and 41 % if the score was greater than 4 points. Maternal cardiac death was rare, occurring in 0.6 % of women, with adverse cardiac events being mostly driven by arrhythmias and congestive heart failure.
When all four risk scores were applied to the CARPREG II study group, the CARPREG II score was found to have the highest discriminatory ability. Even women in the lowest risk group had a 5 % risk of cardiac complications, suggesting there should be a low threshold for assessment by a cardiologist with expertise in pregnancy management.14
Multidisciplinary Care
The care of pregnant women with heart disease involves several stakeholders with different perspectives but common goals: delivery of a healthy baby and a mother free of cardiac complications. This team should include an obstetric anesthesiologist, maternal fetal medicine specialist, nursing staff and a cardiologist with expertise in the management of pregnant women. This group should meet regularly to discuss, anticipate and plan for any difficulties that could arise, as well as discuss strategy for labor, delivery and postpartum care. This multidisciplinary strategy is critical in a field where management often runs in a data-free zone because large randomized controlled trial data are lacking.
Depending on maternal and fetal status, this treatment plan could run along the spectrum of usual labor and delivery procedures with no additional monitoring to labor and delivery in the intensive care unit with invasive hemodynamic monitoring. The early postpartum period is a time of particularly high risk for many reasons, including further auto transfusion from uterine involution and mobilization of dependent edema (increasing preload) and loss of the low vascular resistance placental unit (increasing afterload). Therefore, many women would benefit from being monitored postpartum on a cardiology floor where volume overload can be detected and managed promptly and there is access to telemetry monitoring.
In centers with no clinical expertise on pregnancy management, women can be referred to a specialized center for initial consultation. Recommendations for treatment can then be made, including whether the patient should deliver locally or at a tertiary care center. The advent of telemedicine should make these consultations more convenient for women in remote locations.
Anticoagulation for Mechanical Heart Valves
Women with mechanical heart valves have an elevated risk of complications during pregnancy and only a 58 % chance of a having an uncomplicated pregnancy with a live birth.15 The use of anticoagulants during pregnancy is challenging and influenced by a hypercoagulable state and changes in the volume of distribution and creatinine clearance. Warfarin crosses the placenta and increases the risks of embryopathy, miscarriage and stillbirth, particularly at doses over 5 mg, but has favorable effects for the prevention of valve thrombosis.16 Low molecular weight heparin (LMWH) does not cross the placenta and is therefore safe for the fetus, which suggests that it would be an ideal anticoagulant; however, available data indicates it is linked with a higher risk of valve thrombosis than warfarin.17 This may be related to inadequate and inconsistent monitoring of anti-Xa levels during pregnancy and warrants further investigation.
Current guidelines on the management of anticoagulation during pregnancy are based on retrospective series, many which have a large representation of ball and cage heart valves, which is clearly not compatible with the contemporary landscape of pregnancy-aged women with mechanical heart valves. The current American College of Cardiology/American Heart Association (ACC/AHA) 2014 valve guidelines recommend warfarin during the first trimester of pregnancy if the dose is less than 5 mg or alternatively LMWH or unfractionated IV heparin. Warfarin is recommended during the second trimester with aspirin, regardless of warfarin dose, and then there is a transition to IV unfractionated heparin closer to term in anticipation of labor and delivery.18 The transition period of anticoagulant switch during the first trimester is often the time of greatest risk of valve thrombosis, so careful monitoring during that period is required.15 The ACC/AHA guidelines suggest measuring peak anti-Xa levels with a target of anti-Xa level of 0.8–1.2 U/ml 4–6 hours post dose, but there is data suggesting that, even if peak levels are adequate, the trough level is often subtherapeutic.19
Ultimately, the choice of anticoagulant during pregnancy should involve careful discussion of the risks and benefits of each strategy between the patient and provider.
Acute MI During Pregnancy
Fortunately, pregnancy-associated acute MI (PAMI) is uncommon, although its incidence is increasing and in-hospital mortality remains high at 4.5 %. In a recent analysis of pregnancy-related hospitalizations, 8.1 cases per 100,000 hospitalizations were identified, the majority occurring in the postpartum period. Women with PAMI are older and more likely to have traditional cardiovascular risk factors, as well as gestational diabetes or pre-eclampsia, than those without. In-hospital mortality is much higher in PAMI than in MI not associated with pregnancy, with an adjusted odds ratio of 39.9.20 Although acute MI is uncommon among reproductive-aged women, the risk of acute MI is increased three- to fourfold during and immediately following pregnancy compared to non-pregnant women of the same age.21,22
The predominant mechanism of acute MI during pregnancy is felt to be spontaneous coronary artery dissection (SCAD) and, in the largest series to report angiographic findings of pregnant women who had experienced an MI, SCAD was found in 43 %, 27 % had atherosclerosis, 17 % had coronary thrombosis, 3 % had vasospasm and 3 % had takotsubo cardiomyopathy. An additional 9 % had coronary arteries that looked normal at angiography, which could have represented transient spasm, thrombosis with endogenous lysis or unrecognized SCAD.23 Women with pregnancy-associated SCAD are more likely to have ST segment elevation infarctions, left main or multivessel disease and more marked left ventricular systolic dysfunction than those with SCAD unrelated to pregnancy.24
The diagnosis of PAMI is based on symptoms, an EKG suggestive of abnormalities and cardiac biomarkers. CK-MB levels have been shown to increase following labor and delivery in normal pregnancies and may exceed the upper limit of normal during this period. Troponin, as measured by conventional assays, typically remains normal during normal pregnancy and delivery.25 In one study using a high-sensitivity troponin assay, 4.3 % of women had a troponin level above the upper limit of normal for up to 24 hours postpartum, without any correlation to outcomes, so the significance of this troponin elevation is unclear.26 Until there is further data, any troponin elevation during pregnancy should be further investigated.
The management of PAMI is individualized. However, in the presence of hemodynamic instability, heart failure or refractory chest pain, angiography is warranted. As a high proportion of PAMI cases are caused by SCAD, in which coronary angiography can propagate dissection, methods to reduce coronary manipulation are recommended such as avoiding deep catheter engagement of the coronary ostia and gentle contrast injections.27 Coronary CT may have an emerging role in the population of patients who are at a lower risk with suspected SCAD, and provides additional data on the vessel wall such as presence of plaque, intramural hematoma or dissection.
Women with PAMI who are pregnant should generally be triaged to an intensive care unit with obstetric capabilities and contingency planning for emergent delivery in the event of maternal deterioration.
Medical management in those with PAMI includes usual MI care with some caveats. Thrombolytic agents do not cross the placenta but raise the risks of preterm labor, spontaneous abortion, placental abruption and bleeding.28 Glycoprotein IIb/IIIa inhibitors are not advised during pregnancy or lactation because data is limited, and thienopyridines are not recommended for breastfeeding women.27 Beta-blockers have been associated with fetal growth restriction, but should be used in pregnancy when the benefits outweigh the risk. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, renin inhibitors and mineralocorticoid receptor antagonists are contraindicated in pregnancy. ACE inhibitors can be started post partum and are considered safe during lactation, with preference for benazepril, captopril, enalapril, and quinapril.29 Statins are contraindicated during pregnancy and lactation.
Epidural anesthesia is an important aspect of the care of women as it limits hemodynamic fluctuations during labor and delivery. However, thienopyridines should be not be administered for 7 days before placement of an epidural anesthesia catheter.30 In general, vaginal delivery is preferred for most women with PAMI, and cesarean delivery is generally reserved for obstetric indications.
Congestive Heart Failure
The diagnosis of congestive heart failure (CHF) during pregnancy is challenging, as heart failure symptoms can mimic those of normal pregnancy. Symptoms such as dyspnea, orthopnea, paroxysmal nocturnal dyspnea and peripheral edema do not necessarily represent pathology in pregnant women. Although plasma volume and cardiac output increase during pregnancy, the jugular venous pressure does not, due to increased pulmonary vascular capacitance. Therefore, this is a reliable physical exam tool in the diagnosis of clinical CHF.
One would expect, because of the increased plasma volume and chamber stretch during pregnancy, that the values of B-type natriuretic peptide (BNP) and its amino-terminal pro-peptide equivalent would increase. Current data suggest that they do but they do not rise above the normal range, so they retain their negative predictive value for heart failure in pregnancy.31,32 In one study of 773 healthy women, BNP rose in late pregnancy and the early postpartum period in 6.1 % of these women, who remained asymptomatic and without clinical evidence of cardiovascular pathology.33 Therefore, elevated BNP does not necessarily signify clinically significant cardiovascular dysfunction; however, until supported by further data, an elevated BNP should continue to prompt further clinical evaluation.
Once a diagnosis of congestive heart failure and left ventricular systolic dysfunction has been made, the additional challenge is determining the etiology. Pregnancy can often precipitate clinical heart failure in women with pre-existing but unknown cardiovascular disease, because of the increase in cardiovascular demands on the heart during pregnancy. Clues may be found in a family history of cardiomyopathy, previous pregnancy, later presentation during pregnancy and prior non-pregnant imaging. A dilated cardiomyopathy may be clinically indistinguishable from a new peripartum cardiomyopathy initially. However, women with peripartum cardiomyopathy have a significantly better prognosis over time than those with dilated cardiomyopathy.34
Peripartum cardiomyopathy is defined as the development of idiopathic heart failure and a left ventricular ejection fraction of <45 % occurring late in pregnancy or in the months following delivery. Incidence varies geographically, with pockets of greater prevalence in South Africa, Nigeria and Haiti.35 Management during pregnancy is similar to that for non-pregnant patients, with the caveats of avoiding medications that are harmful to the fetus and decision-making surrounding the timing of delivery. Although the decision is individualized, the principle that a longer gestational age is preferred remains true, especially if maternal cardiovascular status can be stabilized. Pre-term delivery is reserved for maternal instability and cardiogenic shock.
Mode of Delivery
Despite the increased hemodynamic burden of labor and delivery, including a further increase in heart rate, stroke volume and cardiac output, vaginal delivery remains the optimal method of delivery. Cesarean section increases the risk of maternal infection, leads to great hemodynamic shifts and blood loss, brings a risk of surgical injury and raises the likelihood of thrombotic events.12 Although there is no consensus over absolute contraindications for vaginal delivery, cesarean section can be considered for some women with certain cardiac conditions, including preterm labor in those receiving full oral anticoagulation, Marfan’s syndrome with an aorta over 45 mm, acute or chronic aortic dissection, and intractable heart failure. Generally, cesarean section is reserved for obstetrical indications.
Pre-eclampsia and Future Cardiovascular Disease Risk
Pre-eclampsia and hypertensive disorders of pregnancy are a major cause of maternal and fetal death.36 The management of hypertension during pregnancy balances the risk to the fetus (hypotension, effects on fetal growth and medication exposure) and risk for severe maternal hypertension. It is important to note that the goals of lowering blood pressure are not to reduce the risk for pre-eclampsia. A study evaluating tight (diastolic blood pressure 85 mmHg) versus less tight (diastolic blood pressure 100 mmHg) blood pressure control in pregnancy did not show any significant difference in the risks for pregnancy loss, high level neonatal care and overall maternal complications between the groups. Less tight control was, however, associated with a higher frequency of severe maternal hypertension.36 A subsequent meta-analysis of 15 studies demonstrated similar findings.37 The American College of Obstetricians and Gynecologists recommend treating blood pressure >160/105 mmHg during pregnancy to reduce the risk of maternal complications. For pregnant women without end organ damage and blood pressure <160/105 mmHg, pharmacologic antihypertensive therapy is not recommended. For women being treated for chronic hypertension during pregnancy, blood pressure goals are 120–160/80–105 mmHg.38
Although delivery of the fetus and time resolves the clinical findings of pre-eclampsia, there remains potential long-term sequelae to this disorder. Women who have developed pre-eclampsia are at an increased risk of heart failure, coronary artery disease, death from cardiovascular disease and stroke.39 This is particularly true in those who develop early pre-eclampsia and require pre-term delivery.40 It is therefore important that cardiologists inquire about pregnancy history when considering cardiovascular risk factors. Subsequently, they should counsel women with a history of pre-eclampsia to optimize their cardiovascular risk factors and lifestyle. Future studies should clarify the mechanisms surrounding this increased risk and guide strategies for risk reduction.
Conclusion
Cardiovascular disease is a leading cause of maternal death. Cardiovascular training programs are increasingly providing education on the management of cardiovascular disorders during pregnancy and it is increasingly likely that cardiologists will be called upon to manage these women.
There are, however, many unanswered questions on optimal care and clinicians are often working in data-free zones. Fortunately, this field is increasingly being covered at national meetings and research appears to be progressing at a faster pace than in previous decades. It is therefore likely that more data will become available over time on the optimal treatment of these women during pregnancy to improve outcomes for both mother and fetus.