The introduction of percutaneous coronary angioplasty in 1977 by Dr Andreas Grüntzig1 was one of the most remarkable achievements in the cardiology realm, opening the door to numerous advancements in percutaneous coronary interventions (PCIs). Due to advances in PCI techniques over the past four decades, catheters along with four generations of coronary stents have dramatically changed the natural history of acute myocardial infarction (AMI) management and brought hope of improved outcomes to more and more patients. Indications for PCI and demographics of such patients are changing to include increasingly critically ill patients.2 With increasing numbers of high-risk coronary procedures, rapid door-to-balloon times for critical ST-elevation myocardial infarction (STEMI) patients, and expanding PCI indications, cardiogenic shock is becoming a frequent encounter in today’s catheterization laboratory.3
We aim to review adjunct catheterization laboratory devices that support the left ventricular (LV) while undergoing high-risk PCI. Percutaneous LV assisting devices (pLVAD) include: the intra-aortic balloon pump (IABP), the Impella® (Abiomed, Inc), the TandemHeart™ (Cardiac Assist, Inc), the currently investigational HeartMate PHP™ (St Jude Medical) and extracorporeal membrane oxygenation (ECMO). The advancements of each of these devices have helped support patients’ weakened hearts, preparing them for the road to recovery. High mortality and morbidity are closely related to cardiogenic shock following a MI.4–5 However, these tools are critical to the support of thousands of patients each year, allowing many to move on to new therapy or even surgery.
Intra-aortic Balloon Pump
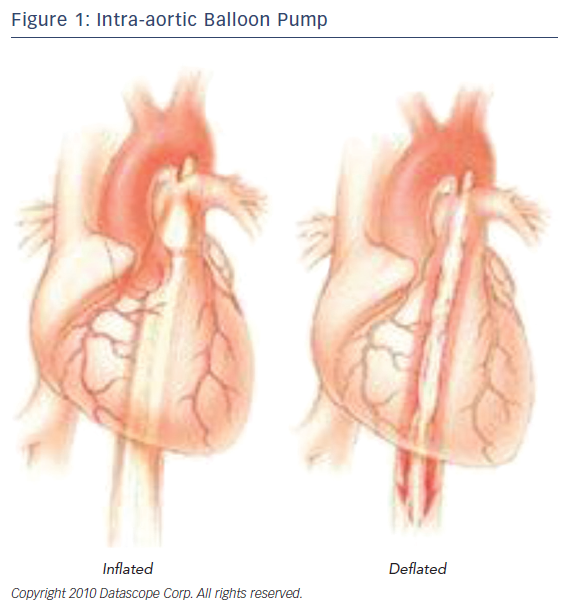
Among the different approaches to the treatment of a failing heart, the intraaortic balloon pump is a frequently used mechanism in medicine today; however, it is an older device in this field of medical technology. It utilizes the idea of counterpulsation, the timing of pressure events to enhance the hemodynamics of the heart. Acting as a circulatory support device, the IABP leads to diastolic augmentation during inflation and potentially contributes to coronary, cerebral, and systemic circulation (Figure 1). In 1967, the first clinical application of an IABP was successfully used on 45-yead-old women that had sustained a MI. By 1976, more than 5,000 patients had received IABP treatment in the US.6,7 Continuous evolution of the IABP led to the development of a percutaneous means of insertion. The adoption of this revolutionary method transformed the field of IABP helping more patients who are unresponsive to other forms of medical management.
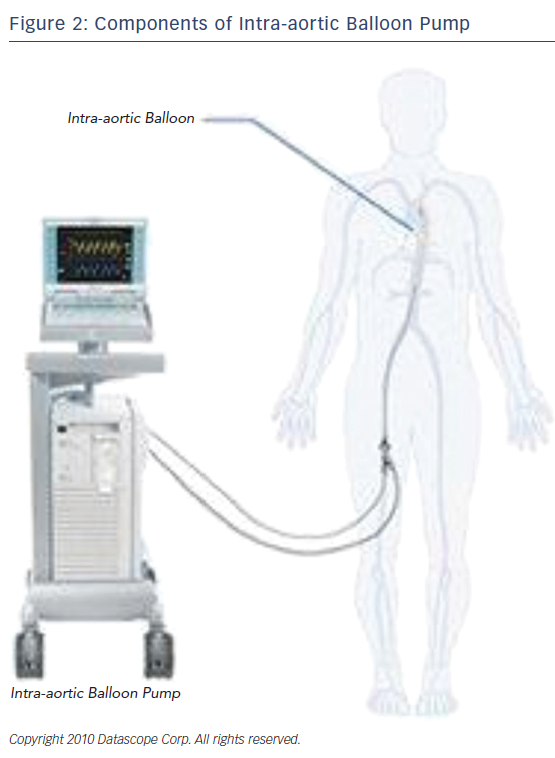
The IABP is a device that consists of a console and a balloon catheter that is filled with either helium or carbon dioxide, typically helium. (Figure 2) Its components include: a gas source, a valve for gas delivery, and a system to monitor blood pressure and acquisition of electrocardiogram. It is very critical that the appropriate size of the catheter is appropriate to the size, age, and weight of the patient. Incorrect sizing or placement can lead to very serious and even life-threatening complications. As for the positioning of the device, the closer the distance between the tip of the balloon and the aortic valve, the greater the diastolic pressure elevation. When considering the types of hemodynamic criteria for mechanical circulatory support one should consider the following: left atrial or right atrial pressure >20 mmHg, urine output <20 ml/h, systemic vascular resistance >2100, systolic arterial pressure <90 mmHg, cardiac index <1.8 l/min, and metabolic acidosis. “Relative exclusion” factors include severe peripheral vascular disease, infection, hepatic disease, stage IV cancer, and severe coagulopathy.9
Despite the widespread use of the IABP, there are still numerous complications that can arise from the use of this device. Ranging from a mechanical error to anatomical risk factors. Cao et al. analyzes some of these risk factors in a study observing the deaths of patients with AMI with IABP support.8 The study consisted of 572 IABP-supported patients with AMI in 72 hours. There were 81 non-survivors and 491 survivors in the study. The most prevalent complication arose from misplaced balloon catheters. Peripheral vascular disease, small size patients, use of sheath of IABP, and diabetes can all lead to difficulty of insertion and mispositioning. A mispositioned pump can augment the blood pressure incorrectly, impair the blood flow of the patient, and lead to infection. The acceptance of less-ideal placement of the balloon was indicative of the morbidity of the patient in most cases.9 The primary complication is arterial damage from the catheter; however, ischemia of the limbs, thrombosis, renal failure, balloon leaks, vessel perforations, and bleeding are all possible complications that can arise.
There are several recent studies done to show the effectiveness of the IABP. A paper from the Society of Thoracic Surgeons observed 88 patients with congestive heart failure who received a subclavian-IABP.10 The goal of the study was to investigate whether the device was an acceptable method to bridge patients to other forms of treatments. Eighty patients could move to their intended goal or desired therapy/surgery. These results concluded this method was an excellent method to improve hemodynamic function, allow for extensive rehabilitation, and permit patients to receive their intended therapy. Although the device is by no means a cure to a patient’s heart failure, it certainly is an efficient approach to begin a road to recovery. Another study delves deeper, and comprehensively studies the effect the IABP has on diastole in 10 patients. Although there is a limitation regarding their small sample size, the researchers concluded IABP counterpulsation has a positive impact on diastolic function in patients undergoing urgent coronary artery bypass grafting (CABG), in addition to the previously-known beneficial effects on systolic dysfunction.11 A much larger study by the Québec Heart–Lung Institute, Laval University performed a meta-analysis utilizing the data of 22 different observational studies, including a total of 46,067 patients. It assessed the effect of IABP on hospital stay and 30-day mortality, while also looking at the length of stay of patients in the intensive care unit and the need for cardiac surgery. The study ultimately shows a significant reduction in mortality rates and length of stay using the IABP. Only 1 % of patients had some form of severe IABP-related complications. They also conclude that among other pLVADs it is the least costly option for patients.12
A well-validated risk prediction score for short-term mortality in cardiogenic shock patients was developed from Intra-aortic Balloon Pump in Cardiogenic Shock (IABP-SHOCK II) trial.13 The score includes six variables: age >73, history of stroke, glucose >191 mg/dl (10.6 mmol/l) on admission, creatinine >1.5 mg/dl (132.6 μmol/l), thrombolysis in myocardial infarction, flow grade <2 after PCI, and lactic acid >5.0 mmol/l on admission. Each variable is assigned 1 or 2 points resulting in three risk categories with a maximum possible score of 9. Low-risk score of <2, intermediate score of 3–4, and a high-risk score of 5–9 correlated with 30-day mortality of 28.0 %, 49.2 % and 76.6 %, respectively (p<0.0001).
Impella
In 1966 the first successful implantation of ventricular-assisting device was performed on a 37-year-old woman by Dr Michael E DeBakey.14 The external circuit could support this patient for 10 days following the operation. This marked the beginning of numerous advancements to the field of ventricular-assist devices. In 1988, Texas Heart Institute introduced “Hemopump”,15 the first small, percutaneously-inserted axial-flow pump via femoral cut-down. The device was not successful commercially; however, it was the precursor to today’s Impella.
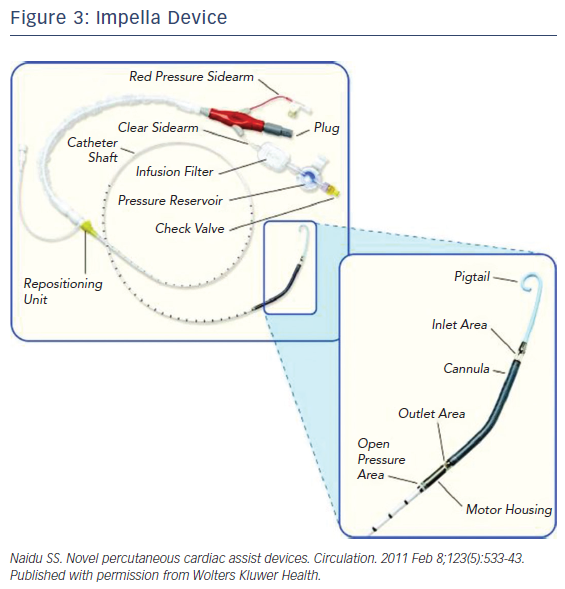
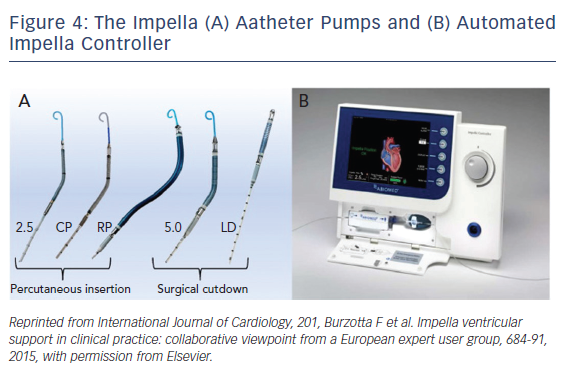
The Impella is a non-pulsatile micro-axial ventricular pump (Figure 3). Its use has been growing since Food and Drug Administration (FDA) approval in 2008 to more than 50,000 patients supported with Impella in recent years. Impella (2.5L and CP) are placed percutaneously via peripheral arterial approach, typically femoral or possibly axillary arteries. However, bigger-sized ones like the Impella 5.0 require surgical arterial cut-down for placement. The pump is available in different sizes allowing for different flow rates and levels of circulatory support. Impella 2.5, CP and 5.0/LD have motors that are 12 Fr, 14 Fr, and 21 Fr with blood flow rates of 2.5, 4.0, and 5.0 l/m, respectively (Figure 4). The motors are mounted on 9-Fr catheters (Impella 2.5, CP and 5.0/LD) with pigtail tip, distal inlet and proximal outlet. The Impella is placed across the aortic valve with the inlet in the left ventricle and the outlet in the ascending aorta. The pump pulls blood from its inlet and expels it into the ascending aorta resulting in unloading of the left ventricle (LV volume dependent), reducing left ventricular end diastolic pressure, decreasing LV work, and myocardial oxygen demand. The pump also increases coronary and systemic perfusion by increasing mean arterial pressure and diastolic pressure.16
Impella RP is designed for right-heart support. Having a similarly designed motor (22 Fr mounted on a 11 Fr), catheter is placed percutaneously with inlet in the inferior vena cava and across the tricuspid and pulmonary valves with outlet in the pulmonary artery bypassing the right atrium and right ventricle.17
The independency from cardiac rhythm gives the Impella advantage over the IABP; however, the need for experience in positioning and troubleshooting of a relatively new device continue to be a major disadvantage of the Impella.
Impella in High-risk Percutaneous Coronary Intervention
After proving safe and potentially efficacious for use in patients undergoing high-risk PCI in A Prospective Feasibility Trial Investigating the Use of the Impella Recover LP 2.5 System in Patients Undergoing High Risk PCI (PROTECT I) trial,17 FDA approved in 2008 the use of Impella 2.5L for partial circulatory support for periods up to 6 hours during cardiac procedures.
The A Prospective Randomized Clinical Trial of Hemodynamic Support with Impella 2.5TM versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention (PROTECT II) trial randomized 452 patients undergoing nonemergent PCI on an unprotected left main or last patent coronary vessel with a left ventricular ejection fraction (LVEF) <35 % and patients with three vessel diseases and LVEF <30 % to Impella versus IABP. The results failed to show superiority of Impella over IABP in reducing adverse events at 30 and 90 days but established Impella as an acceptable and safe hemodynamic support device during high-risk PCI, leading to FDA approval of Impella 2.5 for elective and high-risk PCI in 2015. Recently, in December 2016 the approval was extended to include Impella CP (4 l/min of blood flow) in the same settings.18
Despite failure of the Impella to prove superiority in large multicenter trials as a protective measure in high-risk PCI, several substudies and subanalysis from PROTECT II and registry data suggest more promising results.
A substudy of PROTECT II trial examined the effect of periprocedural MI definition on trial outcomes. The investigators used the validated definition (new Q-waves or a creatine kinase-MB elevation more than eight times normal value) instead of the original protocol definition. Ninety-day major adverse events (MAE) and major adverse cardiac and cerebral events (MACCE) of death, stroke, MI, and repeat revascularization were compared. MAE and MACCE were lower in the Impella group compared with IABP. The study concluded that hemodynamic support with Impella 2.5 compared with IABP during high-risk PCI in PROTECT II trial resulted in improved event-free survival at 90 days.19
A subanalysis of PROTECT II trial evaluated the impact of device learning curve on the outcomes of PROTECT II trial. The analysis included 448 patients at 74 different sites. Among these, 58 patients were the first to receive Impella 2.5 at their site, 62 were the first to receive IABP. Significantly lower 90-day MAE rates were observed with the use of Impella 2.5 compared with the use of IABP after excluding the first patient per group at each site, highlighting the new device learning curve as a potential confounding factor of the trial results.20
Another substudy of PROTECT II evaluated the efficacy of hemodynamic support using Impella 2.5 versus IABP in patients with 3-vessel coronary disease (3VD) and LVEF <30 %. At 30 days after PCI, patients in the Impella group trended toward reduction in incidence of MAE compared with IABP group. Use of Impella 2.5 was an independent predictor of improved 90-day outcomes. Study concluded that patients with 3VD and reduced LVEF show improved outcomes when PCI is performed with Impella 2.5 hemodynamic support.21
USpella registry is an observational ongoing multicenter voluntary registry of Impella use in which 47 sites in the US and two sites in Canada participated. A total of 637 high-risk PCI patients were identified from USpella registry and a further 339 patients who met eligibility criteria for enrollment in the PROTECT II trial, referred to as “PROTECT II-like” group, were identified, and then each of those groups were compared with the 216 patients randomized to the Impella arm of the PROTECT II trial. USpella registry were older, had higher incidence of chronic kidney disease, fewer prior CABG or MI, more prior PCI, more severe heart failure symptoms, and lower LVEF compared with PROTECT II trial patients. Despite higher risk profile, blood transfusions, vascular complications requiring surgery, and mortality were not statistically significant overall in the USpella registry patients and the PROTECT II-like patients when compared with the PROTECT II trial patients. However, vascular complications not requiring surgery where significantly lower in the overall USpella registry, but not the PROTECT II-like patients, when compared with the PROTECT II trial patients. MI was also significantly lower in the USpella registry as was repeat revascularizations concluding that USpella registry patients seem to do better than PROTECT II trial cohort despite having higher-risk profile.22
A retrospective, single-center study was conducted to analyze 230 patients (115 Impella-supported and 115 unsupported matched controls) undergoing high-risk PCI with LVEF <35 %. Primary outcome was incidence of in-hospital acute kidney injury (AKI). Impella support during high-risk PCI was independently associated with a significant reduction in AKI. Despite preexisting chronic kidney disease (CKD) or a lower LVEF, Impella support protection against AKI persisted. The study showed Impella to be protective against AKI during high-risk PCI even in preexisting CKD and decreased EF populations.23
A recently published, retrospective study using cVAD Registry (a global, observational, retrospective, multicenter registry of patients who have been treated with Impella devices) of 36 patients who underwent PCI on an upper left main coronary artery lesion and were supported by a pLVAD (Impella 2.5) found 55.6 % initiated pLVAD support before PCI and 44.4 % initiated pLVAD support after PCI. Overall, survival to discharge was 38.9 %, with patients who initiated pLVAD support before versus after PCI faring better (55 % versus 18.8 %; P=0.041).24
In conclusion, Impella use as a protective measure in high-risk PCI has shown to be non-inferior to IABP in PROTECT II trial. However, more recent substudies and subanalysis of PROTECT II trial, and registries USpella, and cVAD, are showing better outcomes, lower incidence of AKI even in CKD patients, fewer post-procedure MIs, and fewer need for revascularizations.
Impella in Cardiogenic Shock After Myocardial Infarction
The Efficacy Study of Left Ventricular Assist Device to Treat Patients With Cardiogenic Shock (ISAR-SHOCK) was a prospective, two-center, randomized study in cardiogenic shock patient aiming to test whether the Impella 2.5 device provides superior hemodynamic support compared with IABP. The study enrolled 26 patients with cardiogenic shock. The primary endpoint was the change of the cardiac index from baseline to 30 minutes after 30 days. Secondary endpoints included lactic acidosis, hemolysis, and mortality after 30 days. Study established Impella as feasible and safe, and provides superior hemodynamic support compared with standard treatment using IABP.25
The results of the above study lead to wider adoption of Impella use by interventionalists in the setting of cardiogenic shock complicating AMI. In 2014 the largest cohort at the time of USpella registry who received Impella 2.5 in the setting of cardiogenic shock complicating AMI were studied. The study included a total of 154 consecutive patients from 38 US hospitals and reviewed the timing of Impella placement (pre-PCI versus post-PCI) with primary endpoint survival to discharge, and secondary endpoints including assessment of patient’s hemodynamics and in-hospital complications. Both groups were comparable except for diabetes, peripheral vascular disease, chronic obstructive pulmonary disease, and prior stroke, all of which were more prevalent in the pre-PCI group. Patients in the pre-PCI group had more lesions and vessels treated. These patients also had significantly better survival to discharge compared with patients in the post-PCI group (65.1 % versus 40.7 %, P=0.003). The study showed that the initiation of support prior to PCI with Impella 2.5 was an independent predictor of in-hospital survival (OR 0.37; 95 % CI: 0.17–0.79, P=0.01) and concluded that early initiation of the hemodynamic support prior to PCI with Impella 2.5 is associated with more complete revascularization and improved survival in the setting of cardiogenic shock complicating AMI.26
The Impella CP Versus Intra-aortic Balloon Pump in Acute Myocardial Infarction Complicated by Cardiogenic Shock (IMPRESS) trial was a more recent randomized, prospective open-label, multicenter trial assessing whether the Impella CP can decrease 30-day mortality when compared with IABP in patients with severe cardiogenic shock complicating AMI. The trial enrolled a total of 48 patients (24 in each arm). The primary endpoint was 30-day all-cause mortality and results showed that routine treatment with Impella CP was not associated with reduced 30-day mortality compared with IABP. Given such a small study population the trial has limitations and continues to leave several unanswered questions regarding the use of Impella in cardiogenic shock population.27
A recent analysis of cVAD registry concluded that early initiation of hemodynamic support prior to PCI with Impella 2.5, in the setting of AMI complicated by cardiogenic shock was associated with a greater survival benefit to hospital discharge in women compared with men, despite a higher predicted risk of mortality and a greater revascularization failure rate for women.28
As of the date of this review, the FDA-approved indications for the Impella devices are:
- Impella 2.5 and Impella CP for temporary (<6 hours) ventricular support during high-risk PCI performed in elective or urgent, hemodynamically stable patients with severe coronary artery disease and depressed LV ejection fraction as a potential prevention from hemodynamic instability during or shortly after the procedure.17
- Impella 2.5, Impella CP, Impella 5.0 and Impella LD for short-term use (<4 days for Impella 2.5 and Impella CP, and <6 days for Impella 5.0 and Impella LD) and the treatment of ongoing cardiogenic shock that occurs immediately (<48 hours) following AMI.17
The safe use of Impella requires low risk for vascular access complications (the most common complication) and relative structurally normal heart to safely introduce the device through peripheral vasculature, across the aortic valve into the left ventricle. Contraindications for Impella use include LV mural thrombus, significant atrial septal defect or ventricular septal defect, LV rupture, moderate to severe aortic insufficiency (graded as >+2 by echocardiography), mechanical aortic valve, and severe aortic stenosis (<0.6 cm2).17
In a case series of five patients with severe aortic stenosis (valve area of 0.6 cm2) and LVEF of 24 ± 5 % who needed Impella support for PCI, Impella catheter successfully traversed the aortic valve after a balloonassist technique was used in 4 out of the 5 patients. All procedures were well tolerated, with absence of arrhythmia, hypotension, pulmonary edema, stroke, or MI. One patient died 48 hours post-PCI of multiorgan failure. The four remaining patients were well at 30 days.29
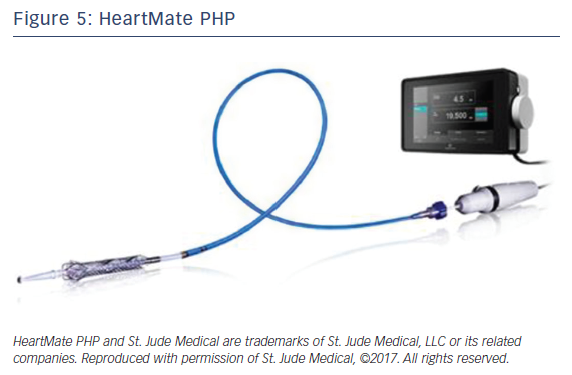
HeartMate PHP
HeartMate PHP is a new percutaneous temporary LV support device manufactured by St Jude Medical currently under investigational use in the US. The device is a low-profile catheter-based heart pump similar to Impella promising to deliver higher blood flow with smaller size compared with Impella.
HeartMate PHP catheter (Figure 5) is inserted over a guide wire through femoral access via a 14 Fr femoral introducer sheath. Once in position across the aortic valve, the outer 14 Fr sheath is pulled back allowing for the 13 Fr cannula to expand to 24 Fr. The cannula has an integrated 3-blade impeller that begins to spin pulling blood from left ventricle into the ascending aorta with physiologic flow rate of 4-5 l/min. Introducing the sheath forward again will collapse the expanded cannula for easy retrieval.
The Coronary Interventions in High-Risk Patients Using a Novel Percutaneous Left Ventricular Support Device (SHIELD II trial) is an ongoing, prospective, randomized, multicenter, open label, noninferiority study comparing HeartMate PHP to the Impella 2.5 in patients undergoing high-risk PCI. The study enrolled 425 patients as of February 2017 and the estimated primary completion date is October 2017 with study completion estimated to be in January 2018. The inclusion criteria included hemodynamically stable patients undergoing elective or urgent high-risk PCI. Patients indicated for revascularization of at least one de novo or restenotic lesion in a native coronary vessel or bypass graft. Complex coronary artery disease was defined as: LVEF <35 % and at least one of the following: last patent coronary vessel, unprotected left main artery, or triple vessel disease. Study excluded emergent PCI, recent history of revascularization in the prior 6 months and STEMI/non-STEMI patients.30
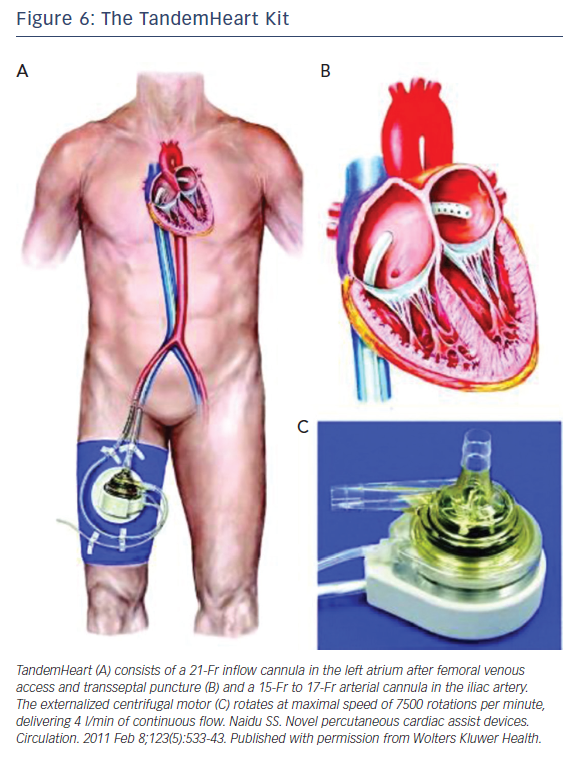
TandemHeart
The TandemHeart Kit is manufactured by Cardiac Assist, Inc. It is an extracorporeal pLVAD that bypasses the left ventricle, reducing LV workload and myocardial oxygen demand. The kit (Figure 6) consists of: (1) an extracorporeal continuous flow centrifugal pump; (2) a trans-septal 21 Fr cannula with a large end-hole and 14-side holes; (3) a femoral arterial line ranging in size from 15 Fr to 19 Fr and is the main determinant of flow rate; (4) a control consoles. It is FDA approved for circulatory support up to 6 hours and also has FDA approval to add an oxygenator to the circuit allowing for concomitant LV unloading and oxygenation.31
The trans-septal cannula is introduced via venous access into the right atrium then across the inter-atrium septum positioning the tip of the cannula into the left atrium to pull oxygenated blood to the extracorporeal pump. The outflow arterial line is placed through femoral line and introduced to the level of aortic bifurcation providing blood at a rate of 3.5 to 5.0 l/min (depending on the size of the outflow arterial line), resulting in reduction in LV preload, LV workload, filling pressures, and myocardial oxygen demand, and increasing arterial blood pressure and cardiac output (CO). TandemHeart function is largely dependent on right ventricular function to maintain left atrial volume. As with Impella, contraindications include severe peripheral vascular disease, profound coagulopathy, bleeding diathesis precluding safe arterial cannulization, major ventricular septal defect, severe aortic insufficiency and left atrial thrombus. The most common complications from TandemHeart use are limb ischemia and major bleeding.
TandemHeart was studied for use in cardiogenic shock cohort after AMI with intended PCI of the infarcted artery. The study randomized 41 patients to either IABP (n=20) or TandemHeart support (n=21). Complications like severe bleeding or limb ischemia were encountered more frequently after TandemHeart support, whereas 30-day mortalities were similar in the two groups.32
TandemHeart started losing popularity likely owing to the need for trans-septal puncture, its high-profile kit and higher complication rate, specifically hemolysis and the need for more blood transfusions compared with IABP.33
Extracorporal Membrane Oxygenation
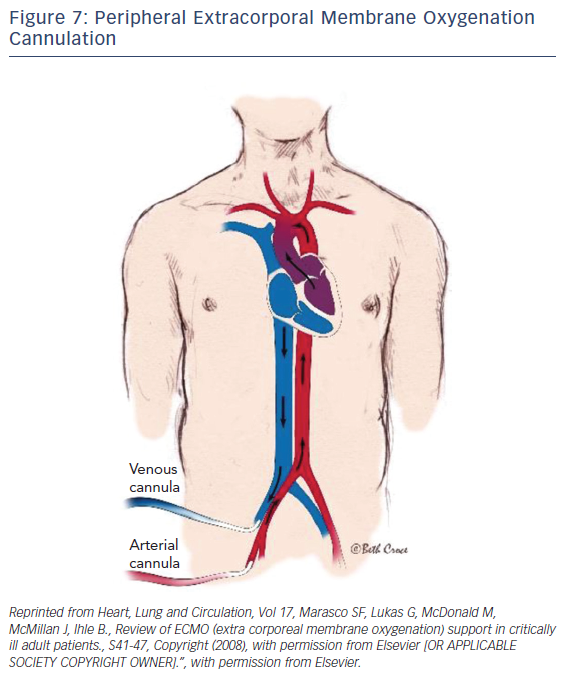
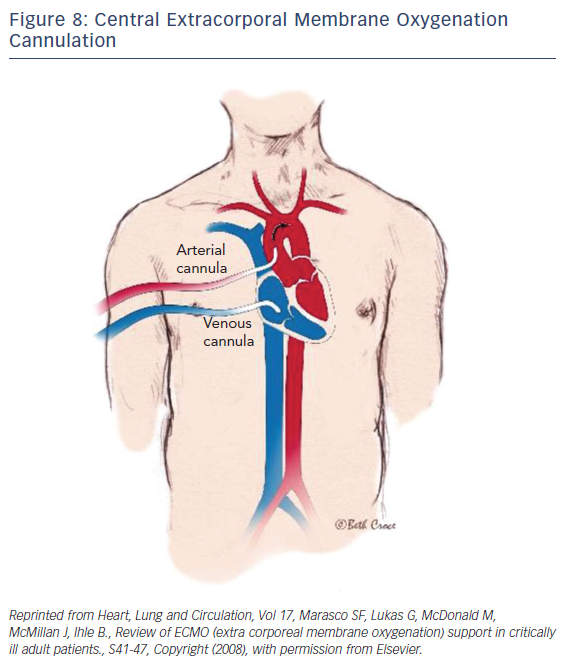
ECMO is a device that externally circulates a patient’s blood providing efficient gas exchange, allowing it to stabilize patients temporarily with heart or pulmonary failure using a semipermeable membrane technology that was first discovered in 1944 by Kloff and Berk.34 Typically, a surgeon would handle the implementation of central ECMO via open approach and peripheral ECMO via a cut-down (Figures 7 and 8). With early technologies, a caretaker could provide a few hours of circulation before hemolysis set in. This barred its use for long-term use; however, in the 1950s the first membrane oxygenator35 was permitted for prolonged cardio pulmonary bypass.35 In the 1960s and 1970s the National Heart, Lung, and Blood Institute organized a nine-hospital collaborative study36 to investigate ECMO therapy; however, errors in the study itself led to inconclusive evidence regarding the improvement of survival rates. Studies during this time reported a 90 % mortality rate with patients afflicted with respiratory failure. After vast technological advancements, ECMO therapy developed enough to improve survival rates up to 75 % in adults with cardiac failure.37 From the 1980s to the early 2000s there was very limited ECMO use due to poor outcomes from prospective studies. Since the early 2000s after much development and analysis there was an increase of ECMO use, utilizing its capabilities as a bridge to transplant or different therapies.
After dramatic evolutionary advancements to the technology, the promising outcomes of Efficacy and Economic Assessment of Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial38 and the role ECMO played in influenza A pandemic,39–40 it became one of the most effective forms of circulatory support. Driven by a rotor unit, the device will drain and return a patient’s blood through a cannula and tubbing. Once the blood is drained, the blood passes through the ECMO device where it is then oxygenated, decarboxylated, and warmed so it can then be returned into the patient. The standard technique of nonsurgical application in adults begins with the peripheral cannulation of the femoral or jugular vessels. The veno-venous ECMO drains and returns blood from the right atrium (Figure 8). Typically, this technique is performed on patients with respiratory distress syndrome. The focus of this paper will be directed towards the veno-arterial (VA) ECMO, which drains blood from the right atrium and returns to the arterial system. The VA-ECMO can reduce the preload of blood and increase aortic flow and end-organ perfusion. Typically, during this treatment, a sheath for antegrade perfusion of the cannulated leg is invoked to prevent leg ischemia. One advantage of this treatment is that the system and technology required is very accessible and mobile, if necessary. Once the device is implanted and operational, the patient can be transported anywhere with the whole unit. The ECMO can establish a right-to-left shunt by draining blood from a vein while returning it to the iliac artery. With large cannulas and rotors, the blood can flow up to 7 l/m, resulting in a significant increase of blood pressure if there is enough vascular resistance (pressure = flow × resistance). Although this device has an extensive variety of benefits and advantages, as is the case with any medical device, complications can arise.
Despite having a quick set-up and sufficient hemodynamic support, the ECMO still has its own list of limitations, contraindications, and complications. Despite having lifesaving capabilities, this device should only be used when patients’ wishes or ethical aspects do not exclude mechanical support. There must be an outlined bridging strategy, or an end goal before the device is implemented. If a patient has severe peripheral artery disease in their iliac arteries, the ECMO may not be a viable option. Other contraindications arise in patients with aortic dissection, relative LV thrombus, or uncontrolled bleeding disorder. Among the complications, leg ischemia may arise so a sheath must be implemented to thwart the threat. Bleeding, LV distention, hyperfibrinolysis and embolism may all occur.41 Other conditions such as two-circulation syndrome and other vascular complications may also arise.42 It is possible to see severe aortic regurgitation caused from the retrograde flow support of the VA-ECMO, leading to the potential event of pulmonary edema or LV distention. Many of these complications occur when caretakers fail to notice specific indications prior to implantation.43
Typically, ECMO is used in patients with compromised ventilation or oxygenation status. If they have suffered a cardiac event such as a MI followed by cardiogenic shock, then implementation of the ECMO may also be used. Usage of the device is also common in cases where there is acute respiratory distress syndrome, viral, bacterial, or atypical pneumonia, barotrauma, and acute chronic interstitial pneumonitis.44 Before the device is used, it should be noted that patients with lifeshortening conditions, such as overwhelming sepsis, non-pulmonary multiorgan failure, irreversible neurological injury, terminal illness, or other life-limiting disease, should not have the ECMO device used. A common candidate for the ECMO may also have hypercapnic, hypoxic, and be unresponsive to optimal medical managements, including lowtidal volumes, bronchodilator treatment, administration of epoprostenol, paralytics administration, and appropriate diuresis.45 Clinical studies show evidence of the effectiveness of this device, depending on proper preprocedural assessments.
Several recent studies pertaining to the ECMO shed some healthy light on the effectiveness of the device and its patients’ outcomes and treatment. In a recent study from the Journal of Cardiac Surgery,46 over 8 years of clinical observations 150 patients were categorized in four groups by specific indications: patients with post-cardiotomy, cardiogenic shock, requiring cardiopulmonary resuscitation (CPR), cardiogenic shock not requiring CPR, and respiratory failure. Among the 150 cases, 73 (41 %) were weaned off ECMO, 40 (23 %) were bridged to their healthcare goal of transplantation or mechanical-assist device insertion, and 63 (36 %) expired on ECMO. Mortality rates and length of stay in the hospital did not vary much between the four groups. The device duration was 4.9 days on average and the stay in the hospital was 40.3 days on average. With a survival rate of 64 % and a relatively small study population, further data to elaborate on the effectiveness of the device can be seen in another study from the Journal of Critical Care.47 Candidates for the study’s population were all high-risk patients in need of CABG surgery. The objective was to prove that ECMO is a viable option to act as a bridge before a large operation. Among their population 100 % received a successful ECMO insertion and 83 % of patients lived the next 6 months’ event free.
The ECMO is a very useful percutaneous device that in some cases can provide a chance for survival or at least a bridge to a definitive treatment. Much research is required before it is clear whether this device reduces mortality.
Conclusion
Despite large, randomized trials failing to show superiority of one device over the other, the IABP seem to be falling out of favor with most interventionalists due to expanding registry data and substudy analysis showing promise in Impella. IABP’s lower CO augmentation (up to 0.5 l/min) and need for regular rhythm are major limitations. With ECMOs being largely managed by the surgeons and the need for septal perforation for TandemHeart placement, Impella offers better CO augmentation compared with IABP, and easier placement compared with TandemHeart. However, Impella is a relatively new device that has a learning curve compared with more established devices as suggested by a substudy analysis of PROTECT II trial.48 Also, technical support and troubleshooting are other considerations when prolonged support is needed.
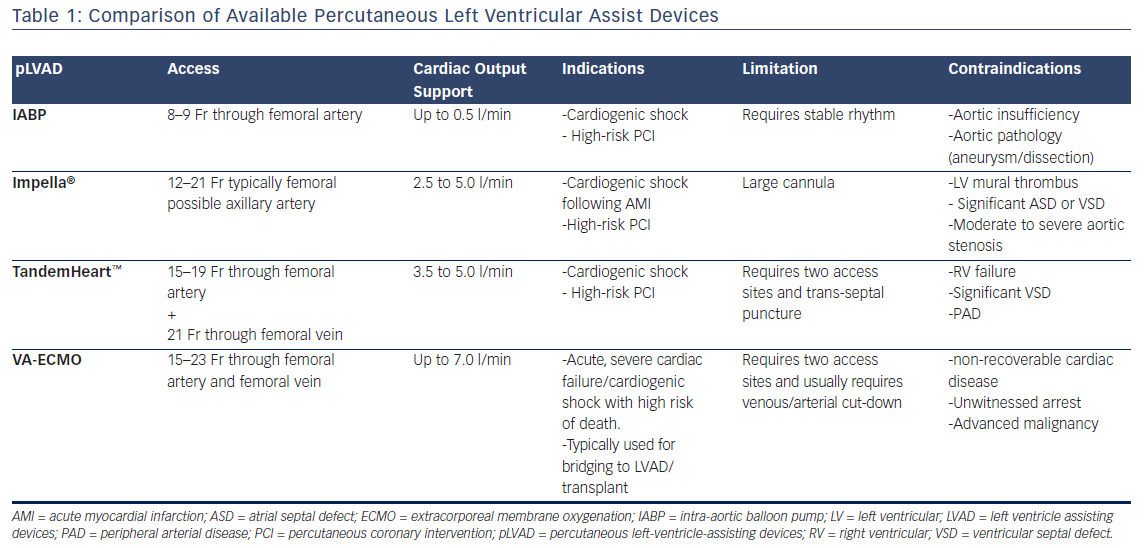
ECMO and TandemHeart have different roles in specific scenarios. Device choice depends on many factors including: patient’s profile (Table 1), nature of procedure (e.g. critical STEMI PCI versus elective high-risk PCI), time limitations, anticipated degree for hemodynamic compromise, anticipated duration of support, availability of the device, interventionalist’s experience with the device, and availability of resources for post-placement management.
There is still much data to be collected and analyzed to determine the best choices in specific clinical scenarios. Each operator and center must determine their needs and skillsets in the setting of current evidencebased data. Clearly, patients benefit from the hemodynamic support in critical cases, whether planned or emergent. Ease of use, cost, and availability of local expertise must also be considered in order to best serve our cardiovascular patients.