Maternal mortality is defined as the annual number of deaths from any cause related to or aggravated by pregnancy, during pregnancy and labor, or within 42 days of termination of pregnancy.1 Pregnancy-related death is defined as death during or within 1 year of pregnancy that was a result of a pregnancy complication or worsening of an unrelated condition due to the psychological effects of pregnancy.2
Global maternal mortality has seen a significant reduction. Between 2000 and 2017, the global maternal mortality rate declined by 38% (from 342 deaths to 211 deaths per 100,000 live births), according to UNICEF.3
While sub-Saharan Africa and South Asia saw the most substantial decreases in maternal mortality, these regions still account for 86% of all maternal deaths worldwide. The low socioeconomic status of these countries, along with limited access to healthcare for many pregnant people, predisposes these regions to higher maternal mortality rates.3
While much of the world has seen reductions in maternal mortality, the wealthiest nation in the world, the US, has experienced a different trend. In the US, maternal mortality has more than doubled in the past three decades, from 7.2 deaths per 100,000 births in 1987 to 17.3 deaths per 100,000 births in 2018.4 The US is the only high-income country to experience such a large increase.
A recent Centers for Disease Control and Prevention study using data from Maternal Mortality Review Committees across 36 US states estimated that 80% of pregnancy-related deaths over 2017–2019 could have been prevented.2 The study found that 7% of pregnancy-related deaths were due to hypertensive disorders of pregnancy (HDP).
More than half (53%) of pregnancy-related deaths happen within 1 year after delivery,2 highlighting the importance of postpartum care and management of conditions, particularly hypertensive disorders, before and long after pregnancy and delivery.
This paper will explore some of the complex reasons behind this increase and propose ways to improve maternal health outcomes as they relate to hypertension during pregnancy.
Prevalence
Hypertension has been noted as one of the leading causes of maternal mortality in high-income countries.5 HDP complicate 5–10% of pregnancies. A review from the WHO identified hypertension as the leading cause of maternal mortality in high-income countries, accounting for 16% of deaths.5
In the US, the incidence of HDP increased from 13% in 2017 to 16% in 2019.2 Preeclampsia complicates about 3% of all pregnancies in the US.6 Healthy women with no history of HDP with a singleton gestation have <10% overall rate of HDP, and are considered low risk.7 However, women with a previous history of HDP or chronic hypertension have a 25–50% rate of HDP onset.7 Therefore, management of high-risk women is important.
Untreated hypertension during pregnancy can result in severe complications for both the fetus and mother. Preterm delivery (birth that occurs before 37 weeks of pregnancy) and low birthweight (when a baby is born weighing less than 5 pounds 8 ounces) are often seen as a result of uncontrolled hypertension during pregnancy. This is due to chronic hypoperfusion of the uteroplacental unit resulting in fetal growth restriction.
Preterm infants have higher rates of mortality and morbidity, particularly hypertension, future cardiovascular disease, insulin resistance, asthma, and neuromotor and cognitive abnormalities.8–13
Untreated hypertension, particularly preeclampsia, can have many consequences for the mother. These women have an increased risk of developing acute sequelae, such as pulmonary edema, renal injury, and posterior reversible encephalopathy syndrome. A history of HDP increases women’s risk of future cardiovascular disease, including MI and stroke, two- to eight-fold compared with those with normotensive pregnancies.14
Pathophysiology
Significant physiological cardiovascular modifications occur during pregnancy to accommodate the growth of the fetus and placenta. Early in pregnancy, higher levels of estrogen, progesterone, and relaxin lead to systemic vasodilation to accommodate the increased blood volume. Additionally, the renin–angiotensin–aldosterone system is upregulated to increase plasma volume. The combination of these leads to increased stroke volume and physiologic anemia (as plasma increases at a faster rate than red blood cells), causing an increase in heart rate to compensate. Both elevated stroke volume and heart rate result in increased cardiac output to maintain blood pressure (BP) and ensure adequate perfusion for both the pregnant person and the placenta.15
Preeclampsia is a multisystemic disease that affects multiple organ systems, requiring immediate and sustained interventions.16 The placenta has been implicated in the pathogenesis of preeclampsia, so the delivery of the placenta was thought to result in the resolution of symptoms. However, postpartum preeclampsia as well as persistence of hypertension beyond placental delivery in some women challenges this hypothesis. An overall reduction in placental perfusion induces systemic vascular endothelial dysfunction and release of reactive oxygen species.17
During a normal pregnancy, the uterine spiral arteries develop into high-capacity blood vessels. In patients with preeclampsia, there is an immunologically initiated alteration in cytotrophoblastic invasion, which leads to improper vascular remodeling of the spiral arteries, ultimately resulting in abnormal blood flow (e.g. high BP and pulsatile flow) and hypoxia of the placenta and fetus.8
Placental hypoperfusion results in a release of vasoactive factors that cause an increase in maternal BP to counteract and ensure sufficient blood supply of the fetus. The release of vascular endothelial growth factor and placental growth factor, among other substances, causes endothelial dysfunction, which results in microthrombosis causing the multiorgan ischemia, oxidative stress, and target organ damage seen in preeclampsia.
Risk Factors
Hypertension in pregnancy has a variety of predisposing factors. Patients with preexisting conditions associated with endothelial damage, including diabetes, renal disease, cardiovascular disease, and hypertension, are at increased risk.8,18–20
Preeclampsia occurs in up to 35% of pregnant women with gestational hypertension and 25% of those with chronic hypertension.21 Those who are obese (BMI >35 kg/m2) have a higher risk of HDP.22
Increasing age is an additional factor, particularly as people are getting pregnant later in life; this raises their risk of having developed comorbidities, such as hypertension, diabetes, and other cardiovascular conditions, as well as the use of assisted reproductive techniques.23,24 Pregnant patients aged >40 years have a maternal mortality rate 4–5 times higher than those in their 20s.25
People with thrombophilias, such as antiphospholipid antibody syndrome, factor V Leiden mutation, as well as protein C and protein S deficiencies, have a higher risk of hypertension during pregnancy.11
Finally, pregnant patients with a family history of preeclampsia and/or hypertension, with lower socioeconomic statuses, and from Black and Hispanic groups have higher rates of HDP.26 A more in-depth discussion on these health disparities is given below.
Definition and Classification
A major challenge in discussing HDP is the lack of precise definitions and nomenclature for the conditions.27 The most current classification of hypertension in pregnancy divides these disorders into six categories: 16,28
- Gestational hypertension is an increase in BP to >140/90 mmHg after 20 weeks’ gestation, with no proteinuria or end-organ damage.
- Preeclampsia is newly diagnosed hypertension with proteinuria (excretion of ≥300 mg of protein in 24-hour urine collection) or end-organ damage after 20 weeks’ gestation. Preeclampsia can occur without hypertension when gestational proteinuria is associated with end-organ damage. End-organ damage impacts the kidneys, liver, brain, and hemostatic systems most often, with hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome being the most profound.29,30
- Preeclampsia with severe features includes a systolic blood pressure (SBP) of ≥160 mmHg or a diastolic blood pressure (DBP) of ≥110 mmHg, platelet count <100 × 103/µl, liver transaminase levels twice the upper limit of normal, a doubling of the serum creatinine level or a level >0.285 mmol/l, severe persistent right upper-quadrant pain, pulmonary edema, or new-onset cerebral visual disturbances.16 An estimated 50% of women with preeclampsia progress to preeclampsia with severe features.31
- Eclampsia occurs when preeclampsia is complicated by new-onset tonic-clonic seizures. These seizures may occur before, during, and after the onset of labor, and are referred to as antepartum, intrapartum, or postpartum eclampsia, respectively. The seizures must not be due to any other cause.
- Chronic hypertension is defined as the presence of hypertension (≥140 mmHg systolic BP and/or ≥90 mmHg diastolic BP) of any cause prior to pregnancy or diagnosed before the 20th week of gestation.
- Preeclampsia superimposed on chronic hypertension occurs if the woman has baseline hypertension before pregnancy or before the 20th week of pregnancy, which is then superimposed with preeclampsia – defined as newly diagnosed proteinuria after 20 weeks’ gestation, sudden worsening of hypertension, and/or signs of end-organ damage.
Current Management Guidelines
The overall goal of treatment is to minimize acute maternal cardiovascular and cerebrovascular complications while maintaining placental perfusion.
There is continued controversy over whether people with mild to moderate chronic hypertension on anti-hypertensive therapy prior to pregnancy should stop these medications, be kept under close observation, and have therapy reinstated for BP elevations of 140–160/90–100 mmHg, versus continuing their usual anti-hypertensive regimen and maintain a BP of <140/90 mmHg.32,33
Currently, both approaches are used and there is insufficient evidence to recommend one approach over the other. The following sections will outline current treatment rationales, summarize recent data suggesting benefit in active treatment of mild chronic hypertension, and highlight the healthcare disparities contributing to the high rates of HDP in the US.
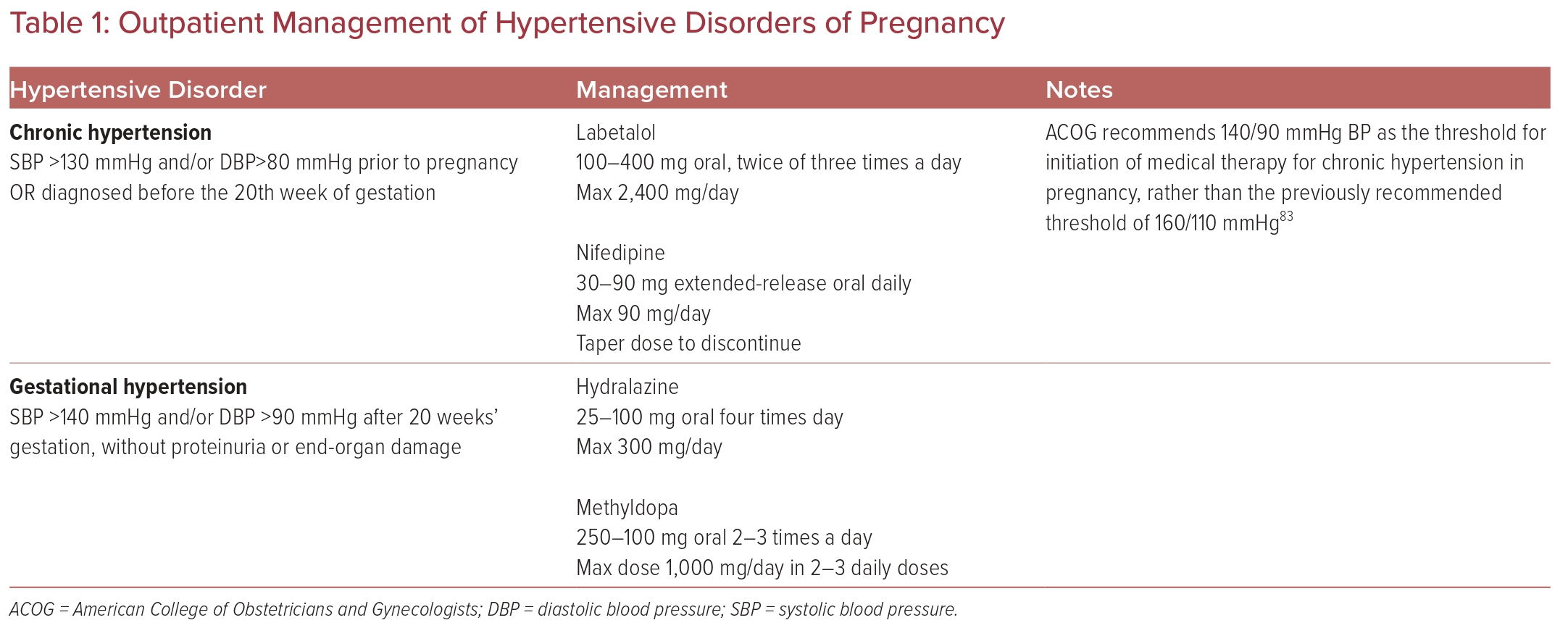
Outpatient Management
Management strategies for hypertension differ, depending on the American College of Cardiology/American Heart Association stage of hypertension.34
Preconception counseling for those with chronic hypertension is key in optimizing maternal-fetal outcomes. Emphasis should be placed on optimizing comorbidities with a focus on smoking cessation, adequate diabetes control, BMI reduction, and chronic hypertension control. All patients with chronic hypertension who are planning a pregnancy should be evaluated with a complete blood count, complete chemistry panel, and urine protein:creatinine ratio to establish baseline renal, liver, and platelet function.
Before pregnancy, clinicians should consider screening for secondary causes of hypertension and carry out a thorough review of medications. Angiotensin-receptor blockers, neprilysin inhibitors, angiotensin-converting enzyme inhibitors, mineralocorticoid receptor antagonists, and sodium–glucose cotransporter 2 inhibitors must be discontinued as soon as the pregnancy is diagnosed or during pre-counseling evaluation when planning pregnancy.
β-blockers can cross the placenta, and could cause fetal growth retardation in the long-term; however, the overall reduction in fetal birthweight attributable to β-blockers is of uncertain clinical significance (<200 g on average) and should be weighed against the potential benefit of β-blocker use when clinically indicated.35 Atenolol has decreased protein binding and increased risk of fetal adverse effects. It is also excreted in breast milk. Therefore, an alternative β-blocker should be used. Labetalol, a nonselective α- and β-blocker, is a safe first-line antihypertensive medication that can be used in pregnancy.16,32 Labetalol should be avoided in those with moderate to severe asthma and abrupt withdrawal should be avoided given the risk of rebound hypertension. Other β-blockers, such as metoprolol and carvedilol, have been used, but less data are available on them, and both drugs are renally excreted, indicating the need for frequent titration during pregnancy.16,32 There are less data for carvedilol than metoprolol.36
While it is reasonable for patients with stage 1 hypertension (SBP 130–139 mmHg or DBP 80–89 mmHg) who were on antihypertensives before conception to continue their medication, the American College of Obstetricians and Gynecologists (ACOG) recommends against the initiation of new medical therapy for newly identified stage 1 hypertension.37 Instead, these patients should focus on healthy lifestyle habits, such as moderate exercise, and alcohol and smoking cessation.
A recent study showed that the Mediterranean diet can reduce the prevalence of preeclampsia, especially in Black women.38 Salt restriction during pregnancy is not currently recommended, given the lack of data about its benefits. However, consumption of non-processed foods with normal sodium content is advised.
The current paradigm of treating hypertension in pregnancy is to use pharmacological therapy only in those with severe hypertension of ≥160/105–110 mmHg, unless there is evidence of end-organ damage (proteinuria, thrombocytopenia, or left ventricular hypertrophy).39 Patients who do not meet these parameters are typically treated conservatively with lifestyle modification. If end-organ damage is present, a lower BP threshold of 150/100 mmHg is recommended.
First-line medications for the treatment of hypertension in pregnancy are labetalol and nifedipine. Second-line agents include methyldopa and hydralazine. Methyldopa is a centrally acting α-2 agonist that has several side-effects, most commonly sedation and headache. One study found that women treated with methyldopa after the first trimester had a significantly increased risk of preterm birth.40 Table 1 summarizes the outpatient management of chronic hypertension in pregnancy and gestational hypertension.
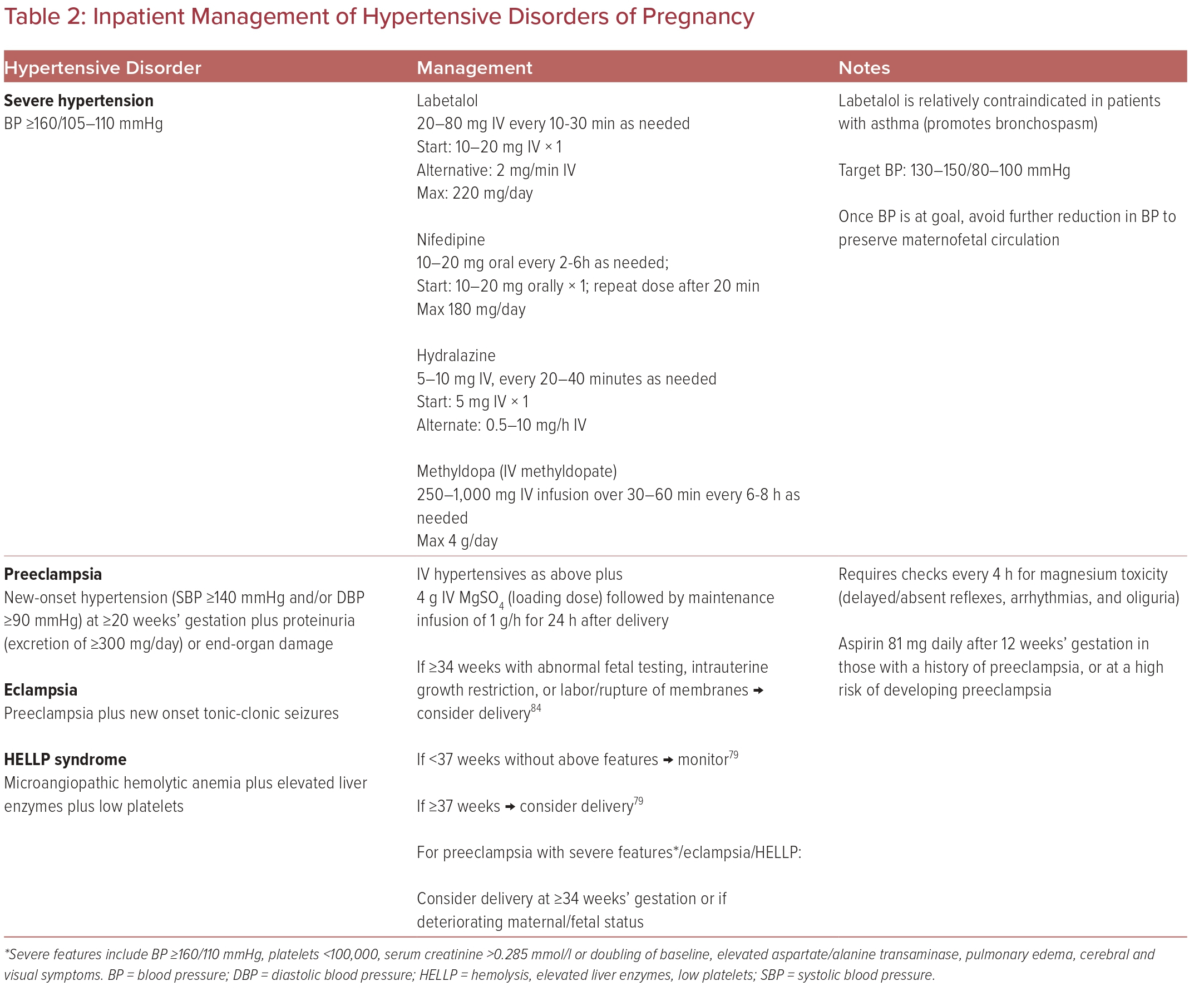
Inpatient Management of Hypertensive Crisis
Patients with severe hypertension (BP ≥160/105–110 mmHg) are further subclassified into persistent, severe hypertensive hypertension (if the severe BP measurement lasts >15 minutes) or nonpersistent (if a non-severe measurement is recorded within 15 minutes).
Since patients with severe hypertension are at an increased risk of stroke, timely recognition of hypertensive crises (BP ≥180/120 mmHg) is essential to prevent maternal fetal morbidity/mortality. To aid in this, in 2019 the ACOG Safe Motherhood Initiative published a hypertensive emergency checklist designed for use in emergency and non-obstetric settings to help providers triage and treat these patients.39
Ideally, treatment of severe hypertension in pregnancy should be initiated within 30–60 minutes of recognition of severely elevated BP. BP should be lowered to achieve the goal of SBP 130–150 mmHg and DBP 80–100 mmHg, which reduces the risk of stroke, congestive heart failure, and death.41 Once at goal, further sudden lowering should be avoided given the risks of maternal cerebral or myocardial ischemia or MI and of reduced uteroplacental perfusion.
These acute severe hypertension episodes should be treated with IV medications, including labetalol, hydralazine, and oral nifedipine. The onset of action of IV labetalol, IV hydralazine, and oral nifedipine is 1–20 minutes, with IV labetalol and hydralazine having the quickest onsets of action of 1–2 minutes and 10–20 minutes, respectively.24 Therefore, patients should have their BP rechecked 10–15 minutes after medication administration. Some sources also recommend concurrent administration of magnesium sulfate (MgSO4) and antihypertensive therapy.9
Table 2 summarizes the inpatient management of hypertensive crisis, preeclampsia, and eclampsia management.
Preeclampsia and Eclampsia Management, Outcomes, and Prevention
Patients who develop preeclampsia are treated as for hypertensive crises with regards to IV antihypertensive medications, with the addition of MgSO4 for seizure prevention. A loading dose of 4 g of MgSO4 is given, followed by a maintenance infusion of 1 g/h, generally for 24 hours after delivery.42 Due to the risk of MgSO4 toxicity, these patients must be monitored every 4 hours to evaluate for delayed and absent reflexes, arrhythmias, and oliguria.25
If BP remains uncontrolled or there is progressive end-organ dysfunction, delivery is often pursued. However, despite delivery, postpartum preeclampsia or persistent postpartum hypertension following pregnancy complicated by HDP are observed in a subset of patients, which merits continued hypertension monitoring for several weeks to months postpartum.43
A systematic review and meta-analysis conducted by Wu et al. in 2017 analyzed 22 studies (published between 2005 and 2015) to determine the long-term cardiovascular outcomes in women with and without preeclampsia.44 They highlighted that a history of preeclampsia-eclampsia was associated with a greater likelihood of long-term cardiovascular disease, including increased risks of chronic hypertension, early onset coronary artery calcification (OR 4.3; 95% CI [1.5–12.2]), heart failure (RR 4.19; 95% CI [2.09–8.38]), coronary heart disease (RR 2.50; 95% CI [1.43–4.37]), coronary heart disease death (aRR 2.63; 95% CI [1.74–3.98]), cardiovascular disease death (aRR 2.21; 95% CI [1.83–2.66]), and stroke (RR 1.81; 95% CI [1.29–2.55]).44–54 Additionally, preeclampsia is associated with an increased risk of peripartum cardiomyopathy that can progress to chronic heart failure.
Because of severe maternal and fetal consequences, patients at high risk of preeclampsia are given low-dose aspirin of 81–150 mg from 12 weeks’ gestation to 36 weeks’ gestation.25 The ASPRE trial showed that treatment with low-dose aspirin in patients determined to be at high risk for preeclampsia following first-semester screening reduced the risk of preterm preeclampsia (<37 weeks) (OR 0.38; 95% CI [0.20–0.74]; p=0.004), with a more pronounced effect on nulliparous women (OR 0.27; 95% CI [0.11–0.65]).55,56 There was no significant difference in the risk of preeclampsia occurring beyond 37 weeks’ gestation or giving birth to a small for gestational age neonate (birthweight below the 10th percentile for gestational age).56
Women who are at a high risk of preeclampsia include those aged >40 years and those with a previous history of preeclampsia (RR 7.19; 95% CI [5.85–8.83]), prepregnancy obesity (RR 2.47; 95% CI [1.66–3.67]), diabetes (RR 3.56; 95% CI [2.54–4.99]), preexisting hypertension (RR 1.38; 95% CI: 1.01–1.87), family history of preeclampsia (RR 2.90; 95% CI [1.70–4.93]), and certain medical conditions such as antiphospholipid syndrome (RR 9.72; 95% CI [4.34–21.75]).42,57
Future Perspectives
Chronic hypertension in pregnancy is not as benign of a disease process as has previously been thought.
Recommended strategies to facilitate non-pharmacological management include using plain, non-medical language, speaking slowly, listening to the patient’s concerns, reinforcing key issues with drawings when applicable, and requesting feedback and teaching back to indicate that the patient understands and, where applicable, their partner.20
As outlined above, the current paradigm recommends avoiding pharmacological treatment of hypertension in pregnancy unless severe hypertension (SBP >160 mmHg or DBP >105 mmHg) develops.
However, a recently published randomized controlled trial, the CHAP study, suggests that treating mild chronic hypertension is associated with better pregnancy outcomes.57 In the study, 2,404 women with mild chronic hypertension and singleton fetuses at a gestational age of <23 weeks were randomized to receive antihypertensive medications recommended for use in pregnancy (active treatment group) or to receive no such treatment unless severe hypertension (systolic BP ≥160 mmHg or diastolic BP ≥105 mmHg) developed (control group). In pregnant women with mild chronic hypertension, active treatment with a BP target of <140/90 mmHg was associated with better pregnancy outcomes than a control strategy of no antihypertensive treatment unless SBP was ≥160 mmHg or DBP was ≥105 mmHg.56 Women who received active treatment had a lower risk of one or more primary-outcome events of preeclampsia with severe features, medically indicated preterm birth at <35 weeks’ gestation, placental abruption, or fetal or neonatal death.56 Therefore, this trial suggests that a strategy of treating mild chronic hypertension can reduce the risk of adverse pregnancy outcomes without increasing the risk of low birthweight for gestational age.
Healthcare Disparities
Healthcare Access
Access to healthcare is essential before, during, and after pregnancy to ensure a healthy pregnancy and to protect the health of the patient.58 It is important that those who are seeking to become pregnant receive preventive care to reduce their chances of developing comorbid conditions, as well as to receive care to manage their existing conditions before conception.
Access to healthcare is impacted by a variety of factors, including socioeconomic status, health insurance, education, geographic location, and race.28,59,60 Access varies widely across the US, with more reproductive healthcare deserts appearing in rural areas. However, a lack of access is also a major issue in metropolitan areas where healthcare resources are abundant but people lack the resources (e.g. money, transportation, insurance, and time) to access them. Additionally, points of entry into healthcare, delayed referral to higher levels of care, documentation, team communication, and a lack of equipment or education influence the quality of care received.
Obstetric complications are higher in hospitals with low delivery volumes.61 Referral of patients with preeclampsia to hospitals with higher obstetric volumes decreases maternal morbidity and mortality because these hospitals have experience of these more complex cases.62 However, 59% of births occur in hospitals with fewer than 1,000 deliveries annually.16 Rural areas particularly face increasing challenges in improving high risk pregnancy outcomes as a result of limited access to healthcare services, financial constraints, patient reluctance to seek healthcare due to cultural or financial constraints, scarcity of services and physicians, health insurance, and limited access to internet services.29
Women in rural areas have higher rates of HDP as well as morbidity and mortality than women in urban areas.30,63 This is partly attributed to limited access to healthcare prior to, during, and after pregnancy, with rural non-Hispanic black and Hispanic women being the most vulnerable.64
Preconception Care
Preconception health is essential to a healthy pregnancy.65 Lifestyle changes, including exercise, management of chronic conditions, and dietary interventions, improve pregnancy outcomes and reduce the risk of preeclampsia.8,32,66
However, access to preconception care is often limited due to a lack of insurance coverage. It is estimated that half of births in the US are covered by public insurance, which is limited to 60 days’ postpartum care and does not include comprehensive preconception care.67 While insurance coverage is essential to accessing care, studies have shown that 25% of women of reproductive age were uninsured at some point in the previous year.68 Black women have the highest rates of being uninsured, so are least likely to have access to preconception care.68 Women who fail to present for prenatal care are at a higher risk of adverse pregnancy outcomes and are more likely to be from minority ethnic groups.69,70
Ensuring that women with preexisting conditions such as diabetes and hypertension are placed on treatment and monitored before they become pregnant is an important way to reduce the incidence of hypertension-related complications during pregnancy.
Prenatal Care
The detection of hypertension during pregnancy is one of the most essential aspects of optimal prenatal care.71 Routine prenatal visits should involve assessment of BP, weight, urine protein, and symptoms suggestive of preeclampsia (e.g. visual symptoms, headaches, epigastric pain, and pulmonary edema).
Accurate BP measurements are essential to the management of pregnancy and to identifying new-onset hypertension during pregnancy. The implementation of out-of-office BP measurements during the COVID-19 pandemic to reduce the clinic volume has resulted in more acute and better predictors of cardiovascular morbidity and mortality.8,72 Self-measured BP in those with chronic or gestational hypertension is an essential strategy to managing changes in hypertension.
Racial Disparities and Hypertension
Racial disparities have a substantial influence on maternal mortality, with studies finding that Black women have a 3-4 times higher risk of pregnancy-related deaths than white, Asian, and Hispanic women.73–75 Studies have found that Black patients are more likely to have preeclampsia and pregnancy-induced hypertension compared to their white counterparts, and they have higher rates of mortality as a result.
HDPs affect more than one in five delivery hospitalizations of Black women and one in six delivery hospitalizations of American Indian and Alaska Native women.76 Individuals from minority ethnic groups, including Black, American Indian, Alaska Native, and Hispanic, are disproportionately impacted by social determinants of health, including systemic racism, which contribute to higher rates of morbidity and mortality than their white counterparts.41,42,44 Specifically, these social determinants may include worse access to preconception care for management of chronic conditions, poorer access to prenatal care during pregnancy, difficulty in acquiring adequate insurance coverage extending into the postpartum period, as well as socioeconomic and environmental factors.41,42,44
Chronic medical conditions that exist prior to pregnancy, such as chronic hypertension, diabetes, thrombophilias, autoimmune disease, kidney disease, and obesity, increase the risk of preeclampsia and eclampsia.41 Black women have higher rates of chronic disease, particularly diabetes, chronic hypertension, and cardiovascular disease.61
Preconception hypertension has nearly doubled from 10.9 to 20.5 per 1,000 live births between 2007 and 2018, with a large proportion of cases found among Hispanic and Black patients.61 Management of HDP is consistently suboptimal for Black, American Indian, and Alaska Native women.77–79 Preconception management of chronic conditions prior to pregnancy may decrease the incidence of both HDP and related complications during and after pregnancy.
Strategies to Improve Health Equity
Addressing these health disparities is essential to reducing maternal morbidity and mortality as they relate to HDP. Improving healthcare access and services at all facilities as well as communication and collaboration between facilities and regional networks are paramount.
In rural settings, incorporating telemedicine for more frequent assessments and transferring pregnant patients with HDP to facilities that can provide timely intervention, are essential to reducing these disparities.44 Management of HDP requires multidisciplinary collaboration among obstetricians, cardiologists, maternal fetal medicine specialists, nephrologists, neonatologists, pharmacists, midwives, nurses, social workers, and many more to ensure effective treatment before, during, and after pregnancy.
Reducing racial disparities among these many specialists and their patients has shown to have an immense impact on the quality of care, treatment, and communication.80 Work must be done to train and hire specialists from all backgrounds to reflect the diversity of those who present with HDP.
Clinicians should be trained to provide culturally and linguistically competent care to instill trust in pregnant patients, particularly as it pertains to HDP, to ensure they return to care and remain adherent to treatment. Additionally, clinical trials and studies must include participants from all racial and socioeconomic backgrounds, particularly Black women, to inform clinical practice and policies, and address maternal health disparities.8
Conclusion
HDP are leading causes of maternal morbidity and mortality across the US. Rates are exacerbated by limited access to preconception and prenatal care, maternity care deserts, racial disparities, and late initiation of treatment.
While much of the literature recommends avoiding pharmacological treatment of hypertension in pregnancy unless severe hypertension (SBP >160 mmHg or DBP >105 mmHg) develops, a recent study questions this approach and suggests a possible benefit of treating mild chronic hypertension to improve pregnancy outcomes.50 More research is needed to delineate the risks and benefits of treating all types of HDP as well as chronic hypertension in pregnancy.
Improving access to preconception, prenatal, and postnatal care is essential to reducing and/or managing comorbidities, identifying HDP early, initiating appropriate treatment, and overall reducing future heart disease for patients with gestational capacity. With 80% of maternal mortalities being identified as preventable, these interventions are necessary to improving pregnancy-related outcomes in the US.2
Regular follow-up of these patients by primary care medicine or cardiology services during the postpartum period and for life is necessary to mitigate and address cardiovascular risk factors that can occur prematurely in patients with a history of HDP.81,82