Functional or secondary tricuspid regurgitation (TR) refers to TR that occurs secondary to conditions that cause right ventricular (RV) and/or right atrial dilation, such as left-sided heart disease, chronic AF, or pulmonary hypertension in the absence of organic lesions of the tricuspid valve apparatus. Approximately 80–90% of all TRs are functional and up to 30% of patients undergoing cardiac surgery for left-sided valve disease present with significant TR. Although most commonly seen in conjunction with mitral valve disease, it is also frequently associated with aortic valve pathology, particularly aortic stenosis.1
While the landscape of cardiac interventions has been largely transformed by advancements in transcatheter procedures, the management and understanding of TR, especially in the context of other valvular diseases, continues to evolve. This article aims to delineate prevalence and clinical implications of TR after transcatheter aortic valve replacement (TAVR) and review percutaneous tricuspid interventions.
Pathology and Prevalence
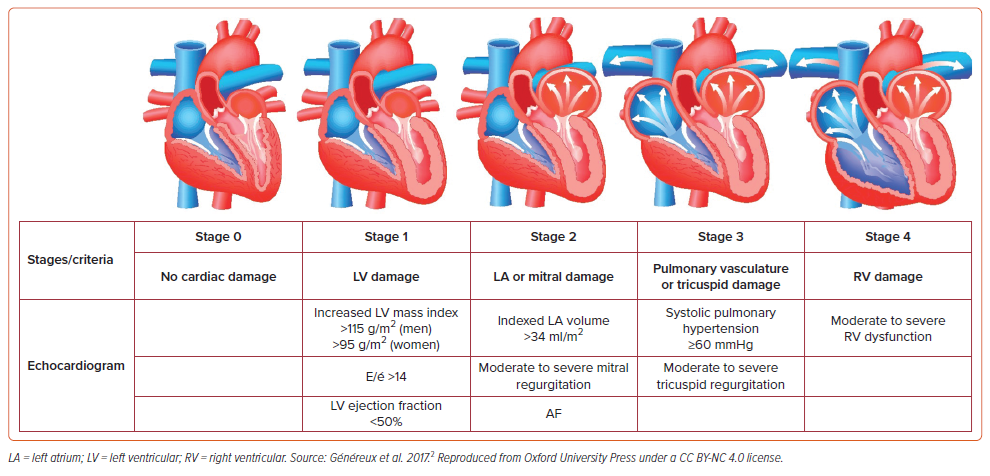
TAVR has emerged as a cornerstone procedure for patients with aortic stenosis, offering a less invasive alternative to surgical valve replacement. For patients who have undergone TAVR, the extent of cardiac damage at baseline and its change at 1 year has important prognostic implications. Généreux et al. developed a staging system to classify the amount of cardiac damage from aortic stenosis (Figure 1).2 The pressure overload created by severe aortic stenosis leads to a cascade of retrograde cardiac changes including left ventricular (LV) hypertrophy, left atrial enlargement with elevated filling pressure, AF, pulmonary hypertension, RV remodeling, and TR. TR is a relatively common finding in patients with aortic stenosis, particularly in stage 3 and stage 4 cardiac damage, with moderate or greater TR reported in up to 27% of patients undergoing TAVR.3–8
In many cases, there is a reduction of TR after TAVR that follows the relief of pressure overload; however, in about 5–7% of cases, TR persists and progresses after TAVR.5,9 There are two proposed mechanisms for the lack of improvement: increasing preload and persistent diastolic dysfunction.
With the first, the relief of the aortic valve gradient increases stroke volume, which increases systemic venous return and RV volume load. For patients with RV dysfunction, the right ventricle is unable to augment systolic function proportionately and dilates with increased preload allowing further tricuspid leaflet tethering and annular dilatation, and the TR worsens.
Although TAVR can reduce LV afterload, the degree of diffuse interstitial myocardial fibrosis may persist.10,11 This persistent fibrosis results in diastolic dysfunction, increased LV end-diastolic pressure, and ultimately post-capillary pulmonary hypertension. Persistent pulmonary hypertension leads to RV enlargement and dysfunction and worsening TR. A meta-analysis by Tang et al. demonstrated that post-capillary pulmonary hypertension is associated with increased mortality as it is more susceptible to permanent myocardial damage and irreversible pulmonary vascular remodeling compared to pre-capillary or combined pulmonary hypertension.12 This assumption that late secondary TR after a left heart valve procedure develops because of post-capillary pulmonary hypertension is supported by Dumont et al.’s study as patients with severe TR after TAVR had more prominent left atrial dilation and higher estimated pulmonary artery pressures.1
Implications of Post-TAVR Tricuspid Regurgitation
Several studies have explored the clinical ramifications of concurrent TR in individuals who are undergoing TAVR. Although a baseline presence of TR was associated with increased risk for mortality after TAVR, the impact was not significant after multivariable adjustment for clinical and echocardiographic variables.
Various studies have shown progression of baseline TR to severe or greater post-TAVR is associated with a fourfold increased risk of all-cause death (HR 4.08; 95% CI [1.92–8.67]; p<0.001), a two- to threefold increase risk of cardiovascular death at 1 year (HR 2.07; 95% CI [1.02–4.21]; p=0.045), a fourfold rate of hospitalization (HR 4.08; 95% CI [1.64–4.93], p<0.001), and persistent functional impairment at 1 year.3,5,8,13 To support this, multiple studies have demonstrated that the lack of improvement in TR following TAVR was reported as a predictor of mortality.2,5,14 Therefore, persistent TR after TAVR may have more important clinical implications than TR before TAVR.
Because of this, predicting which TAVR patients may have persistent or progressive TR might help guide heart failure management and consideration for future tricuspid valve intervention.
Predicting Post-TAVR Tricuspid Regurgitation
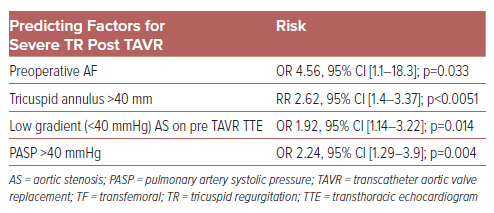
Preoperative AF was repeatedly observed as a statistically significant risk factor for lack of improvement in TR post TAVR (OR up to 4.56).1,6,8,14–16 While it is often difficult to determine if AF leads to TR or worsening TR begets AF, the association is strong. AF induces atrial remodeling and subsequent tricuspid annular dilation and is a known marker for advanced cardiac dysfunction.8 Other factors of persistent TR following TAVR include tricuspid annulus >40 mm (RR 2.62; 95% CI [1.4–3.37]), low gradient (<40 mmHg) severe aortic stenosis on pre-TAVR transthoracic echocardiogram (OR 1.92; 95% CI [1.14–3.22]; p=0.014), and systolic pulmonary artery pressure ≥40 mmHg (OR 2.24; 95% CI [1.29–3.9]; p=0.004), have been identified as strong predictors (Table 1).1,8
Medical Treatment of Tricuspid Regurgitation
There are minimal guidelines on medical regimens specifically for TR. Proven guideline-directed medical therapies to treat the primary etiology of heart failure should be the first priority. These may include vasodilators for pulmonary arterial hypertension, guideline-directed medical therapy (GDMT) for LV systolic dysfunction, and emerging guideline therapies for heart failure with preserved LV ejection fraction as indicated.17 In heart failure with reduced ejection fraction, therapies include angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), angiotensin receptor neprilysin inhibitors, β-blockers, mineralocorticoid corticoid antagonists (MRAs), and sodium glucose cotransporter 2 inhibitors (SGLT2i). In heart failure with preserved ejection fraction, data now supports the use of MRAs and SGLT2i for symptomatic relief.18 Although not included in GDMT, diuretics may ameliorate TR associated with chronic congestive heart failure and fluid overload. While MRAs or ACEI/ARBs have well-known benefits for LV remodeling, the effect on RV remodeling or functional improvement in patients with severe functional TR has not been validated in clinical studies.
Surgical Treatment of Tricuspid Regurgitation
Although current guidelines recommend concomitant tricuspid valve surgery in patients with severe TR undergoing surgical aortic valve replacement (class 1, level of evidence b-nr), there is no recommendation on the management of concomitant TR in patients undergoing TAVR.17 Many patients are at high risk for perioperative mortality following tricuspid valve surgery with in-hospital mortality rates of 8.2%.19,20 Patients who are at high surgical risk with persistent symptoms of right heart failure with TR despite maximally tolerated medical therapy can be considered for transcatheter tricuspid valve interventions (TTVI).
Transcatheter Tricuspid Valve Interventions
There are a growing number of transcatheter treatments developed for TR. These can be categorized as orthotropic, where the valve is deployed at the tricuspid valve annulus, or heterotropic, where valves are deployed in one or both venae cavae. Orthotropic TTVI includes edge-to-edge repair, annuloplasty devices and valve replacements. Heterotopic TTVI includes caval valve implantation.21 Most devices currently have preclinical or early feasibility data; however, we will focus on a few of these where imminent US commercialization is anticipated due to evidence from pivotal trials demonstrating that they are safe and effective therapies. These studies of treatment for severe, massive, or torrential TR also report findings to suggest favorable hemodynamic changes and right-sided heart remodeling following transcatheter tricuspid valve repair or replacement. Below, we focus on TTVI devices that are in advanced stages of investigation and are already or may soon become clinically available in the US.
Orthotropic TTVI
Edge-to-Edge Repair
Transcatheter edge-to-edge repair (TEER) uses a percutaneous method, usually through the femoral vein, to bring together sections of tricuspid valve leaflets using a deployed device. This action aims to reduce TR by effectively sealing the regurgitant orifice. The implant is visualized with echocardiographic and fluoroscopic guidance. The TriClip (Abbott) transcatheter tricuspid valve repair system consists of a 25 Fr delivery guide catheter with steering designed for a tricuspid valve approach. Through this TriClip guide, a MitraClip (Abbott) device (as popularized for the treatment of mitral regurgitation in the past decade) can be used to approximate the tricuspid valve leaflets with its two device arms.22
The PASCAL system (Edwards Lifesciences) is another edge-to-edge repair device family adapted from mitral regurgitation repair for the treatment of TR. This device is made up of two folding paddles that work with clasps to allow independent leaflet capture and bring leaflets into a central 10 mm nitinol spacer or narrow central component (PASCAL Ace).23 The system also consists of a 22 Fr steerable guide sheath, steerable catheter, and an implant catheter. Rotational knobs on the external handles of the guide sheath and steerable catheter allow flexion movements. Leaflets are usually grasped individually but can be obtained simultaneously. When both paddles are closed, the residual TR grade and transvalvular gradient are evaluated before final deployment. If the result is suboptimal, the implant could be reopened, repositioned or removed, if necessary, before final deployment.
A prospective randomized study by Sorajja et al. in 2023 enrolled 350 patients with an average age of 78 years comprised of about 55% women.24 Prior cardiac or transcatheter therapy was noted in about 40% of the patients, with prior aortic valve intervention occurring at an equal rate – about 15% – between the treatment and control groups. About 25% of patients had been hospitalized for heart failure in the preceding year before enrollment. Of patients who could be evaluated, 71% had TR categorized as massive or torrential. There were significant baseline pathological changes of the right heart with a mean annular diameter of the tricuspid valve of 4.4 ± 0.7 cm and a mean right atrial volume of 148 ± 84.3 ml. Among the patients randomly assigned to the TEER group, the device was successfully implanted in 98.8% of patients, with a mean of 2.2 ± 0.7 clips used per patient. The mean device time from insertion to removal was 90 ± 66 minutes, with a median length of hospital stay of 1.0 day, and 97.7% of patients were discharged to home. There was one death in the TEER group (0.6%) judged to be not related to the device or procedure. There was a marked reduction in TR in the TEER group, with 87% of patients having moderate or less severe TR at 30 days and the reduction appeared to be stable at 1 year follow-up. Although there was no significant difference in survival or hospitalization for heart failure between the TEER and control groups, the study demonstrated a statistically significant improvement in quality of life, with a mean increase in 12.3 ± 1.8 points on the Kansas City Cardiomyopathy Questionnaire (KCCQ) score in the TEER group, compared to 0.6 ± 1.8 points in the control group (p<0.001).24
The lack of apparent differences between the groups in these endpoints may have been affected by rigorous patient selection that resulted in enrollment of patients with fewer overall comorbidities than previous studies, as well as rates of death and heart failure hospitalization that were lower than predicted when the trial was designed. In other words, this may have been an overall ‘healthier’ group of severe TR patients for whom long-term outcomes of torrential TR without treatment with a device may become more apparent over time. Subgroup analyses comparing the two groups by OR on measures of right atrial volume, tricuspid annular plane systolic excursion (TAPSE), central venous pressure, mean pulmonary artery pressure, and cardiac output, all favored the TEER device group.
Additional studies of edge-to-edge repair for severe TR have further supported device safety and efficacy, as well as favorable right-sided hemodynamics and remodeling following device placement. The CLASP TR trial was a single-arm, multicenter, prospective early feasibility study designed to test safety and efficacy of the PASCAL transcatheter valve repair system for patients with severe or greater tricuspid regurgitation and persistent symptoms despite medical treatment.25 Sixty-five patients were enrolled in the trial, with a mean age of 77.4 years, 55.4% women, 97% with severe to torrential TR (baseline severity was moderate in 3.1%, severe in 28.1%, massive in 29.7%, and torrential in 39.1%), and 46% with New York Heart Association (NYHA) functional class III or IV. Of the enrolled patients, around 17% had prior aortic valve surgery or intervention. The study demonstrated high survival and low complication rates for the PASCAL device. The system had 91% and 98% implant and procedure success rates, respectively, with 46.2% of patients receiving one device and 41.5% of patients receiving two devices over a median implantation time of 118 minutes. There were six unsuccessful implants attributed to complex anatomy (severe tethering, complex chordal structures, and/or large papillary muscles). There was reduction in TR severity to moderate or less in 70% of participants at 30 days (p<0.001) which was sustained and increased to 86% moderate or less at 1 year. All patients who received a device achieved one or more grade improvement in TR severity, and 75% of those patients achieved an improvement of two or more grades at 1 year. In addition to improvements in TR severity, there were also improvements in functional status and quality of life at 30 days which were sustained at 1 year. There was a low 1-year mortality rate of 10.8%, and heart failure hospitalization rate of 18.5%, which showed a relative reduction of 56.4% when comparing site-reported preprocedural hospitalizations. Importantly for this discussion, this study’s comparison of echocardiographic parameters at baseline and 1 year suggested favorable right-sided heart remodeling. There were significant reductions in tricuspid annulus diameter, RV end-diastolic diameter, TR jet area, right atrial volume, and inferior vena cava diameter.26 The larger, randomized CLASP II TR pivotal trial is currently enrolling patients to further investigate this.
Tricuspid Valve Replacement
The EVOQUE system (Edwards Lifesciences) uses a transfemoral approach and consists of a 28 Fr percutaneous delivery system, a trileaflet bovine pericardial tissue valve, nitinol frame, and fabric skirt. Nine anchors are positioned between the chordae tendineae to engage and capture native leaflets while the valve frame and fabric skirt minimize the risk of paravalvular leak.25
The TRISCEND II trial evaluated safety and efficacy of this transcatheter tricuspid valve replacement (TTVR) system in patients with severe TR who are high-risk surgical candidates with suboptimal anatomy for TEER, such as large coaptation gaps, very dilated annulus, and pacing leads that are pinning leaflets open. The trial had a two-part design, providing early safety and efficacy findings for the first 150 patients at 3 months, 6 months, and 1-year follow-up under the Food and Drug Administration’s breakthrough devices program. The trial enrolled patients with at least severe TR, with evidence of right-sided heart failure associated with severe TR (with exclusion of those with severe RV dysfunction and pulmonary hypertension) receiving optimal medical therapy (OMT) at the time of assessment. Patients were randomized 2:1 to EVOQUE or OMT. Published data from the first 150 patients demonstrated an elderly, comorbid patient population at high-to-extreme surgical risk with poor baseline functional status and quality of life (mean age 79 years, about 80% women, 75% NYHA functional class III or IV, mean KCCQ <20 points, 91% with AF, and 20% with ascites). Baseline TR severity was massive or torrential in 55% of patients, and severe in the others.
Of all enrolled patients, 22 patients (39.3%) had previous left-sided valve surgery or intervention. There was successful device implantation in all but four patients, with a mean procedure time of 115 minutes and a mean device time of 65 minutes. The median length of hospital stay was 4 days, but more than 90% of patients were discharged home. The rate of major adverse events was 27.4%, which was less than expected (43.8%). There was significant reduction in TR grade to moderate or less in 98.8% of patients, to mild or less in 93.8%, and to none/trace in 77.8%. In addition to the primary efficacy outcome of TR grade reduction, the study also evaluated a hierarchical composite endpoint comprised of KCCQ score improvement of 10 points or greater, NYHA functional class reduction of one or more, and a 30 m or greater improvement in 6-minute walk distance. At 6 months, patients with EVOQUE had a highly statistically significant improvement in the composite endpoint, with a win ratio of 4.6 over OMT.
Coprimary outcomes of 1-year follow-up data in 27 patients showed sustained improvement in NYHA functional class and improvement in the degree of TR. Echocardiographic findings were compared at baseline, 30 days and 1 year, demonstrating progressive reduction in RV end-diastolic dimension and inferior vena cava diameter.27 These findings again indicate the capacity for positive right-sided heart remodeling response to reduction of TR severity. A concern with TTVR is the potential for acute RV failure following device implant due to a sudden loss of pressure ‘pop-off’; therefore, patients with severe pulmonary hypertension were excluded. In this study, 30-day echocardiogram findings showed an early reduction in RV systolic function with a subsequent trend for recovery indicated by RV fractional area change and TAPSE, suggesting that despite 45% of patients having massive or torrential TR, the loss of a ‘pop-off’ valve was tolerated.
Other TTVIs
Tricuspid valve annuloplasty involves the use of transcatheter suture or ring implantation techniques to reshape and reduce the size of the tricuspid annulus. There are a few tricuspid valve annuloplasty devices, such as Cardioband, Trialign, and TriCinch, that have completed early clinical testing however, they have not yet reached successful pivotal trials.
Heterotropic TTVI
Transcatheter caval valve implantation (CAVI) refers to the heterotopic placement of a transcatheter valve into the vena cava to restrict the backflow from the failing tricuspid valve. Devices such as TricValve, Trillium, and off-label use of Edwards’ Sapien valve systems have various degrees of clinical evidence but are also still seeking to complete successful pivotal trials in the US and elsewhere.
TTVI and TAVR
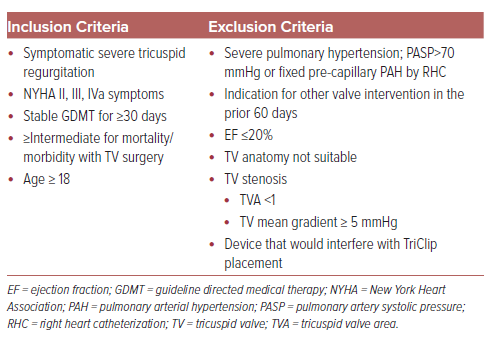
There are no clinical trials of combined TAVR and tricuspid interventions, but as seen by the patient demographics in the TRILUMINATE, CLASP TR, and TRISCEND II trials, it is common that patients being evaluated for TTVI have had prior aortic valve intervention. One study used a prospective TAVR registry to document prevalence and severity of TR before and after TAVR, quantify potential eligibility for TTVI, report clinical outcomes as a function of severity of TR and potential candidacy for TTVI.5 Following TAVR, patients were considered potential candidates for TTVI based on inclusion criteria of the TRILUMINATE trial with having either moderate TR and an NYHA functional class greater than or equal to III, or severe or massive TR and NYHA functional class ≥ II (Table 2). After TAVR, 63 patients (3.1% of eligible database participants) were deemed potential candidates for TTVI. Patients considered candidates for TTVI following TAVR had a twofold increased risk for all-cause and cardiovascular death between 30 days and 1 year after TAVR (HR 1.93; 95% CI [1.15–3.25]). At 1 year, 37.2% of the surviving potential candidates for TTVI had persistent NYHA III or IV symptoms, compared to 11.5% of those who did not qualify (adjusted RR 2.80; 95% CI [1.78–4.40]). Interestingly, post-TAVR TR, but not baseline TR, was significantly associated with an increased risk of mortality. The findings of this study, though limited by size and retrospective analysis, found a significant proportion of patients with symptomatic moderate or worse TR following TAVR had an increased risk for mortality and functional impairment at 1 year. Given the findings of the previously discussed trials demonstrating significant improvement in TR grade and functional status following transcatheter tricuspid valve repair and replacement, TTVI after TAVR may be a reasonable consideration to improve outcomes in the appropriate populations.
Future Directions
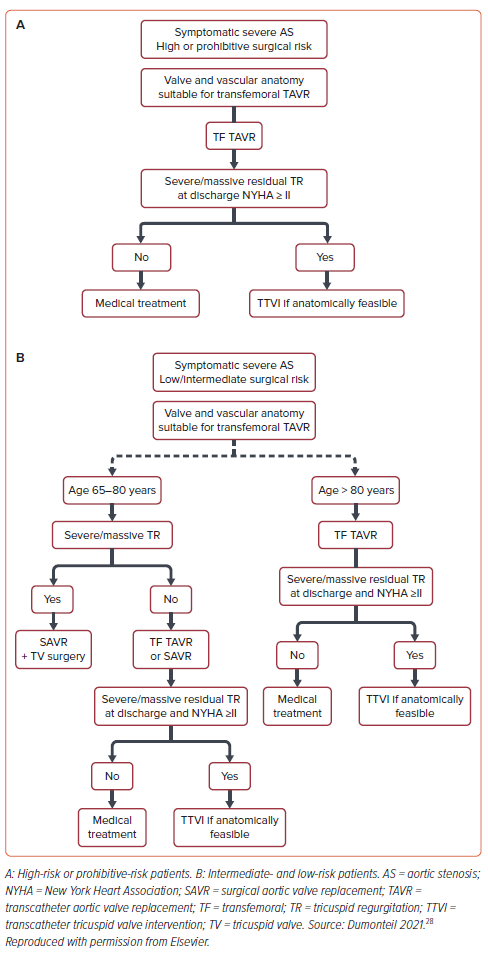
Although the degree of TR improves in 93–95% of patients after TAVR, an area of focus could be timing of TTVI after TAVR in the <7% of patients in whom TR does not improve despite maximally tolerated diuretics and GDMT. As highlighted before, patients with symptomatic, severe TR after TAVR have a twofold increase in all-cause mortality and cardiovascular death between 30 days and 1 year after TAVR. An editorial by Dumonteil suggested a speculative treatment algorithm for such patients (Figure 2).28 Although it is feasible to perform TTVI at the same time as TAVR, it would not be advantageous to perform simultaneously because most patients have an improvement in TR after TAVR.28
Overall, treatment of heart failure in the setting of multivalve pathology needs further innovation. Experience shows that medical therapy alone may alleviate some functional regurgitant valve lesions (mitral or tricuspid) however, this is not always sufficient. Future efforts to predict, prevent, and reverse cardiomyopathies will be necessary to provide non-invasive amelioration of functional TR.
Earlier recognition and treatment of aortic valve disease may become standard of care as TAVR becomes safer, more durable, and more widely available. Treatment of moderate aortic valve disease is being investigated in randomized trials such as PROGRESS (NCT04889872), TAVR UNLOAD (NCT02661451), and EXPAND TAVR II (NCT05149755). If the results of these trials are favorable, we may find that the incidence of progressive cardiac damage and TR also decrease.
Implantable electronic devices in the right ventricle are commonly found in patients with severe TR. Such devices can directly damage or impinge on the valvular apparatus to cause regurgitation after implantation. Alternatively, many implants may not cause severe regurgitation acutely but, in the setting of progressive heart chamber dilatation from other causes, the implant position may negatively impact valve function over time. While a small proportion of these electronic implants may be the primary etiology of tricuspid valve dysfunction, many more are inconvenient bystanders at a minimum, whose presence could physically interact with TTVIs, obstructing this treatment option. Development of less obtrusive implantable electronic devices and increased surveillance of their potential contribution to tricuspid valve dysfunction will be important.
While a small proportion of these electronic implants may be the primary etiology of tricuspid valve dysfunction, many more are inconvenient bystanders at a minimum, whose presence could physically interact with TTVIs, obstructing this treatment option.
Unfortunately, there is little to no evidence to suggest that secondary TR will be effectively prevented in the near future therefore, structural solutions will continue to be needed. Despite decades of work, major improvements in the safety profile of open-heart surgical techniques for TR have not materialized. Transcatheter techniques offer this improved safety profile as discussed above, but continued innovation and refinement is needed. Given the anatomical variability, complexity of the tricuspid valve and many comorbidities that these patients often have, there is unlikely to be a one-size-fits-all TTVI. Continued clinical trials of the multiple categories of TTVIs described above will be important to relieving heart failure symptoms and improving patients’ quality of life.
Conclusion
Individuals presenting with left-sided heart disease such as aortic stenosis often suffer from retrograde propagation of cardiac damage. As this cardiac damage progresses, severe functional TR presents added dysfunction exacerbating the heart failure syndrome which can persist even after treatment of left heart failure. There is growing clinical evidence that transcatheter tricuspid valve interventions can be used as therapeutic options for symptomatic patients with severe TR worldwide. Further investigations will be necessary to define best clinical practices in patients with multiple cardiac pathologies.
Clinical Perspective
- Transcatheter tricuspid valve intervention (TTVI) after transcatheter aortic valve replacement (TAVR) is an emerging field within interventional cardiology, which is being driven by the increasing recognition of concomitant tricuspid valve disease in TAVR patients.
- TTVI encompasses various techniques, including tricuspid valve repair, replacement, annuloplasty, and edge-to-edge repair aimed at addressing tricuspid valve pathologies in patients who have undergone TAVR.
- TTVI after TAVR holds clinical significance as untreated tricuspid valve disease can affect patient outcomes and quality of life, necessitating comprehensive evaluation and intervention strategies.
- Challenges in this field include patient selection criteria, anatomical complexities, varying pathologies, optimal timing for TTVI, procedural complexities due to previous TAVR devices and the need for further research to establish best practices and improve patient outcomes.
- Collaboration between cardiologists, interventionalists, and cardiac surgeons is essential in evaluating and treating residual TR after TAVR with medical therapy alone or TTVI, ensuring that patients receive comprehensive care and optimal treatment for their valvular pathologies.