The concept of the multidisciplinary heart team has existed for many years in various formats, but its role became more accepted and formalized upon the arrival of the SYNTAX coronary revascularization trial.1 This trial demonstrated, to a wide audience, the feasibility and value of a team approach between cardiologists and cardiac surgeons in modern practice. In the trials leading to the approval of transcatheter aortic valve replacement (TAVR) by the Food and Drug Administration (FDA), patients were required to be assessed by both cardiologists and cardiac surgeons, thus further fostering the collaborative decision-making model ushered in by the SYNTAX trial.2–6 The reimbursement requirements by the Center for Medicare and Medicaid upon the first TAVR approval in 2011 (whereby an interventional cardiologist and cardiac surgeon must jointly participate during the evaluation and valve placement to be eligible for payment) have helped to sustain the role of the heart team.
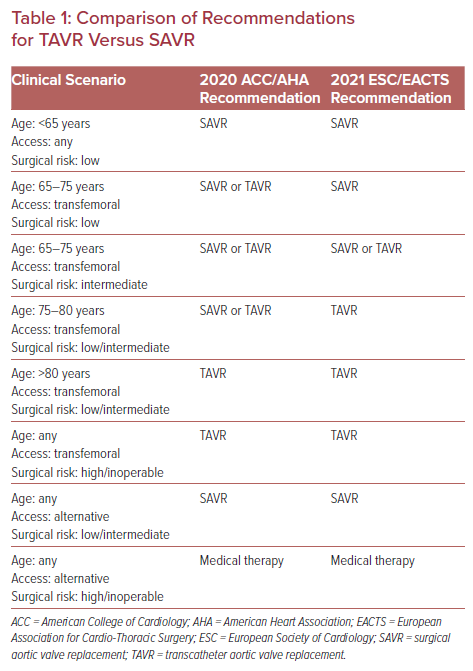
Initially, TAVR was commercially available only to older patients at prohibitive operative risk.7 In that setting, the heart team’s main role was to evaluate a patient’s anatomical candidacy due to the limitations of the TAVR technology with regards to available valve sizes and modes of delivery. However, as TAVR devices have improved and studies have shown that TAVR is both a safe and effective treatment strategy when compared with surgical aortic valve replacement (SAVR) for patients of all levels of surgical risk, clinical decision-making regarding the use of this technology has become infinitely more complex.2–6,8,9 Although the updated American College of Cardiology/American Heart Association (ACC/AHA) guidelines (released in 2020) and updated European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines (released in 2021) provide a valuable framework for the management of patients in a rapidly changing field (Table 1), there remain several areas where evidence is lacking and the guidelines are unable to make major recommendations.10,11 Accordingly, the heart team, which has expanded to now include echocardiographers, radiologists, heart failure specialists, cardiac anesthesiologists, intensivists, nurses, and social workers, has never been more important.
Heart Team Augmentation of the Guideline Recommendations for the Treatment of Aortic Stenosis
For most patients presenting with aortic stenosis (AS) requiring aortic valve replacement (AVR), the first major decision remains the mode of valve replacement (surgical or transcatheter procedure). In line with its previous iteration, the 2020 ACC/AHA guidelines recommend SAVR for patients younger than 65 years old or for patients with a life expectancy >20 years.10 SAVR also remains the preferred modality over TAVR for those patients in whom vascular or valvular anatomical features are deemed unsuitable for transfemoral TAVR, due to the less favorable outcomes associated with alternative-access approaches and particular valve morphological features (e.g. heavy left ventricular outflow calcification, low-lying coronary arteries).10 Conversely, for patients at prohibitive or high surgical risk with a life expectancy of >12 months, patients at low or intermediate surgical risk who are >80 years of age, or patients with a life expectancy of <10 years, TAVR is recommended.10
Although societal guidelines are able to offer a clear framework regarding the roles of TAVR and SAVR for certain patient populations (e.g. the young, relatively healthy patient or the very old patient with several comorbidities), for many patients the guidelines are less definitive and even conflicting. Nowhere is this uncertainty more obvious than when examining the recommendations for patients between the ages of 65 and 80 years at low or intermediate surgical risk. In this population the ACC/AHA guidelines give a class 1 indication for either SAVR or transfemoral TAVR.10 Conversely, the 2021 ESC/EACTS guidelines recommend SAVR for patients <75 years old who are deemed at low risk for surgery, TAVR in patients ≥75 years of age of any surgical risk or in patients of any age at high surgical risk, and either SAVR or TAVR in patients between the ages of 65 and 75 years at intermediate surgical risk.12
While there are several reasons for these somewhat different recommendations, the unknown durability of the TAVR implant is certainly a contributing factor to the more conservative recommendation of the ESC/EACTS to consider SAVR in low-risk patients <75 years old. Although the most current data suggest that the TAVR device has similar durability compared with SAVR implants for up to 5–8 years of follow-up, whether rates of valve failure will diverge beyond this timeframe remains unknown.13–15 For those patients who do outlive their initial TAVR implant, their options include either repeat TAVR-in-TAVR or TAVR explant followed by SAVR. In appropriately selected patients, early data have shown that TAVR-in-TAVR is relatively safe with a low risk of mortality, stroke and coronary obstruction.16 Indeed, the ability to manage TAVR valve failure via a transcatheter route is particularly appealing, given the high risk of mortality associated with TAVR explant and surgical AVR.17 That said, there are numerous anatomical variations, particularly involving sinus width and depth and coronary heights, that, in combination with the initial choice of valve implant, may limit the ability to perform future TAVR-in-TAVR.
Assessment of Surgical Risk
Given that the ACC/AHA or ESC/EACTS guidelines offer differing age cut-offs for SAVR versus TAVR and neither document directly addresses the issue of preferred initial valve implant when planning for future procedures, the heart team is thus responsible for evaluating a patient’s surgical risk and counselling AS patients on the matters of valve durability and the complexities of subsequent valve procedures. Although several risk scores exist for the prediction of outcomes after TAVR and/or SAVR, including the Society of Thoracic Surgeons Predicted Risk of Mortality score and EuroSCORE II, it is widely accepted that available risk models in this area have significant limitations.18–24 In general, risk scores perform best in the population from which they were derived. As such, differences in care patterns across geographical areas or the evolution of procedural care over time can result in decreased model calibration and subsequent poorer prediction of risks associated with a certain procedure. Furthermore, a risk score derived from a population of surgical patients may not function as well in a population of patients being treated with a percutaneous procedure. Additionally, patients at the extremes of the model’s population (e.g. those at very high risk) are underrepresented by definition, which results in poorer risk score performance in these patients. In total then, risk scores derived from a SAVR population may not be particularly accurate when applied to patients receiving TAVR, due to a combination of the difference in the procedures as well as to the fact that many TAVR patients have substantial comorbidities (and are often inoperable) and thus are poorly represented in the surgical populations.25–27 Although some of these limitations have been overcome by developing TAVR mortality prediction models in a population of TAVR patients, the discrimination of these risk scores still remains moderate at best, and this is likely to be due to certain clinical and anatomic factors (e.g. frailty, dementia, left ventricular outflow calcification, right ventricular dysfunction, and low-lying coronary arteries), which affect treatment strategy and outcome but which are not included in many risk models.20,22–24 In accounting for these additional variables, the heart team acts to augment the available risk prediction models, and thus is able to provide a more nuanced and refined calculation of the risks associated with each treatment strategy in an individual patient.
Shared Decision-making
If a patient still remains a candidate for either TAVR or SAVR after anatomical and surgical risk assessment, then the patient’s preference for one treatment or the other should be assessed by the heart team using a formal shared decision-making process. Shared decision-making encompasses the process in which patients and providers engage in a bidirectional exchange of information, which includes a discussion of the clinical issue and the available therapeutic options while taking into account all clinical evidence and the patient’s preferences, so that a treatment strategy, optimized for the individual patient, can be agreed upon. This process can include the use of a patient-centered decision aid tool, of which there are several specifically designed for the management of AS, although these tools are not solely sufficient to form a complete shared decision-making process.28,29 Given that studies have shown that physician and patient preferences do not always align, it becomes even more important to thoroughly assess the values and goals of treatment of the AS patient, especially given that many patients with severe heart failure have been shown to prioritize improved quality of life and other patient-centered outcomes over more traditional clinical outcomes of mortality.30,31 Additionally, patients may place different weights on the varying risks of specific complications (e.g. need for permanent pacemaker), uncertainty regarding valve durability, recovery times associated with SAVR versus TAVR or possible complexities of a future valve procedure. As such, the heart team carries a significant role in participating in the shared decision-making process with the patient in order to better tailor the treatment plan to the individual patient’s preference.
That said, despite multiple studies demonstrating that shared decision-making is associated with better patient knowledge and expectations about their treatment options and the fact that shared decision-making is strongly recommended in both the 2020 ACC/AHA and 2021 ESC/EACTS guidelines, shared decision-making has been shown to be under-utilized both by heart teams and in general clinical practice.10,11,32–36 Focused research has shown that limited physician understanding of the true concept of shared decision-making as well as limited experience with the use of decision aids has impeded the broader implementation of shared decision-making in actual practice.35 In an effort to understand the prevalence of shared decision-making in a real world setting, the Transcatheter Valve Therapy (TVT) Registry now collects data on whether shared decision-making was used and if any specific decision aid was applied. Additionally, there are ongoing trials to evaluate the optimal framework to improve the use of shared decision-making processes and decision aids in the treatment of AS.37
Benefits of the Heart Team Beyond the Individual Patient
Beyond the proposed benefit to the individual patient, the heart team may also be beneficial to clinicians and the health system. With medicine and the cardiac patient population becoming more complex due to the increasing variety of therapeutic options and the greater burden of comorbidities, the heart team permits the pooling of knowledge and expertise, ensuring that the treatment is tailored to the patient using the latest evidence. Any one individual practitioner is unlikely to be adequately skilled in the entire spectrum of clinical, investigative and technical aspects of all of the latest treatment options, and the combination of expertise in a heart team allows for the optimal managing of complicated clinical scenarios, while also facilitating innovation.38,39 This opportunity to interact and collaborate with colleagues to provide optimized care can improve knowledge, skill sets and job satisfaction for the individual clinician.39
At the health system level, the heart team approach can lead to a more effective use of healthcare resources. Indeed, economic analyses have demonstrated that the overall cost effectiveness of TAVR compared with SAVR has improved over time. Although TAVR was initially more expensive than SAVR, TAVR has now been shown to be an economically dominant strategy for the treatment of patients at low and intermediate surgical risk when compared with SAVR.40–42 Furthermore, recent real world analyses have demonstrated that contemporary TAVR is less costly than SAVR in an all-comer population.43 Given that the cost of the TAVR prosthesis has not changed substantially over the last 10 years, the decrease in cost associated with TAVR has been due primarily to the shorter length of stay as compared with SAVR. While there are many contributing factors to the improved cost-effectiveness of TAVR, the development of more protocolized and streamlined patient assessment and care through the collaboration of multiple providers has certainly led to a decrease in resource usage. For example, the Vancouver 3M (Multidisciplinary, Multimodality, but Minimalist) approach is a clinical pathway involving many members of the heart team (e.g. cardiology, anesthesia, nursing, social work, physical therapy), which is aimed at permitting next-day discharge after TAVR. Not only has this pathway been shown to be associated with good clinical outcomes at low-, medium- and high-volume TAVR centers, it has also been shown to be associated with an economic benefit to the hospital system as well.44,45
The Future of the Heart Team
Although the heart team has been shown to be beneficial in the cardiac arena, a decrease in heart team usage during the evaluation process for TAVR has been noted over time.46-48 Certainly, as the management of valvular heart disease at experienced, high-volume centers becomes more protocol-driven, it is possible that full heart team involvement for procedural planning may not be absolutely essential for every AS patient. That said, as TAVR continues to be applied to younger patients, decision-making will extend beyond merely the choice of TAVR or SAVR, but will also include the choice of a particular TAVR prosthesis, the implantation technique, and consideration for future transcatheter interventions. These factors may have prognostic implications over the patient’s lifetime and thus should be considered when tailoring the initial procedure, not only by the medical professionals, but also by the patient themselves. With an increasing need for patient involvement in care decisions, the traditional weekly heart team meeting may need to evolve to include a more organic, patient-centered, bedside discussion. In the current healthcare landscape, this is likely to involve enhanced reimbursement for consultative time with patients and improved clinician training in shared decision-making skill sets, given that both issues have been suggested to factor as perceived barriers to the heart team achieving its full potential.35 However, although team-based assessments have been suggested to be associated with improved care, the question of whether the costs incurred to encourage a more patient-centered, evolved heart team will be economically attractive to a healthcare system remains unanswered.49,50
Conclusion
The landscape of valvular heart disease treatment has changed dramatically in the past decade, and continues to evolve rapidly. The rise of transcatheter valve technology has shifted treatment paradigms and the decision to pursue TAVR versus SAVR for AS has become increasingly complex, thereby requiring an accurate assessment that extends beyond simple eligibility for a particular procedure. The multidisciplinary heart team has matured to become a valuable tool in the management of structural heart disease and continues to play an essential role in the care of the patient with AS. As the field of transcatheter valvular technology continues to advance, the heart team will need to evolve to address the ever growing needs of a complex patient population, faced with nuanced treatment decisions.