Cardiovascular disease affects more than one-third of American adults and is the leading cause of mortality in the United States and worldwide.1 Only 4.5 % of those over the age of 20 meet the ideal levels of the seven metrics of cardiovascular health including cholesterol levels.1 Of modifiable risk factors, including smoking, hypertension, diabetes, and obesity, dyslipidemia has been shown to be the most strongly associated with myocardial infarction (MI).1,2 Numerous epidemiological studies have demonstrated that cardiovascular risk increases significantly as low-density lipoprotein cholesterol (LDLC) increases.3,4 Cholesterol-lowering therapies are thought to be primarily responsible for the reduction in deaths due to coronary heart disease in the United States over the past few decades, and there is a clear association between LDL-C-lowering therapies and improved global outcomes from cardiovascular disease.5–8 The management of dyslipidemia continues to be the cornerstone of primary and secondary prevention of cardiovascular diseases and is a major focus of recent and ongoing study.
In this article, we will review the evidence for lifestyle and pharmacological therapies for dyslipidemia and will summarize and compare the current major societal guidelines for cholesterol-lowering therapies for the primary and secondary prevention of cardiovascular disease, with particular focus on recommendations for appropriate patient selection for LDL-C-lowering therapies for primary prevention.
This review is based on a literature search performed in PubMed for articles published between 1980 and 2016, using combinations of the following terms: cholesterol, hyperlipidemia, dyslipidemia, cardiovascular disease, atherosclerosis, coronary artery disease, treatment. References within the obtained publications were also reviewed.
Treatment Strategies
Lifestyle Modifications
Lifestyle modifications have been shown to lower serum cholesterol levels, with the most notable benefits coming from diet and weight loss. Dietary strategies to improve cholesterol include reducing cholesterol intake to <200 mg daily and reducing total fat intake to <20 % of total caloric intake. Additionally, the inclusion of dietary soluble fiber, phytosterol esters, soy isoflavones, and nuts have all been shown to reduce LDL-C, in most cases by 5–10 mg/dL.8 Physical activity does not reduce LDL-C independent of weight loss, but has been shown to improve cardiovascular health through other mechanisms, and is a cornerstone of weight loss. Overall, through weight loss, reducing dietary cholesterol and fat intake, LDL-C can be lowered by approximately 10–15 %.9
Statins
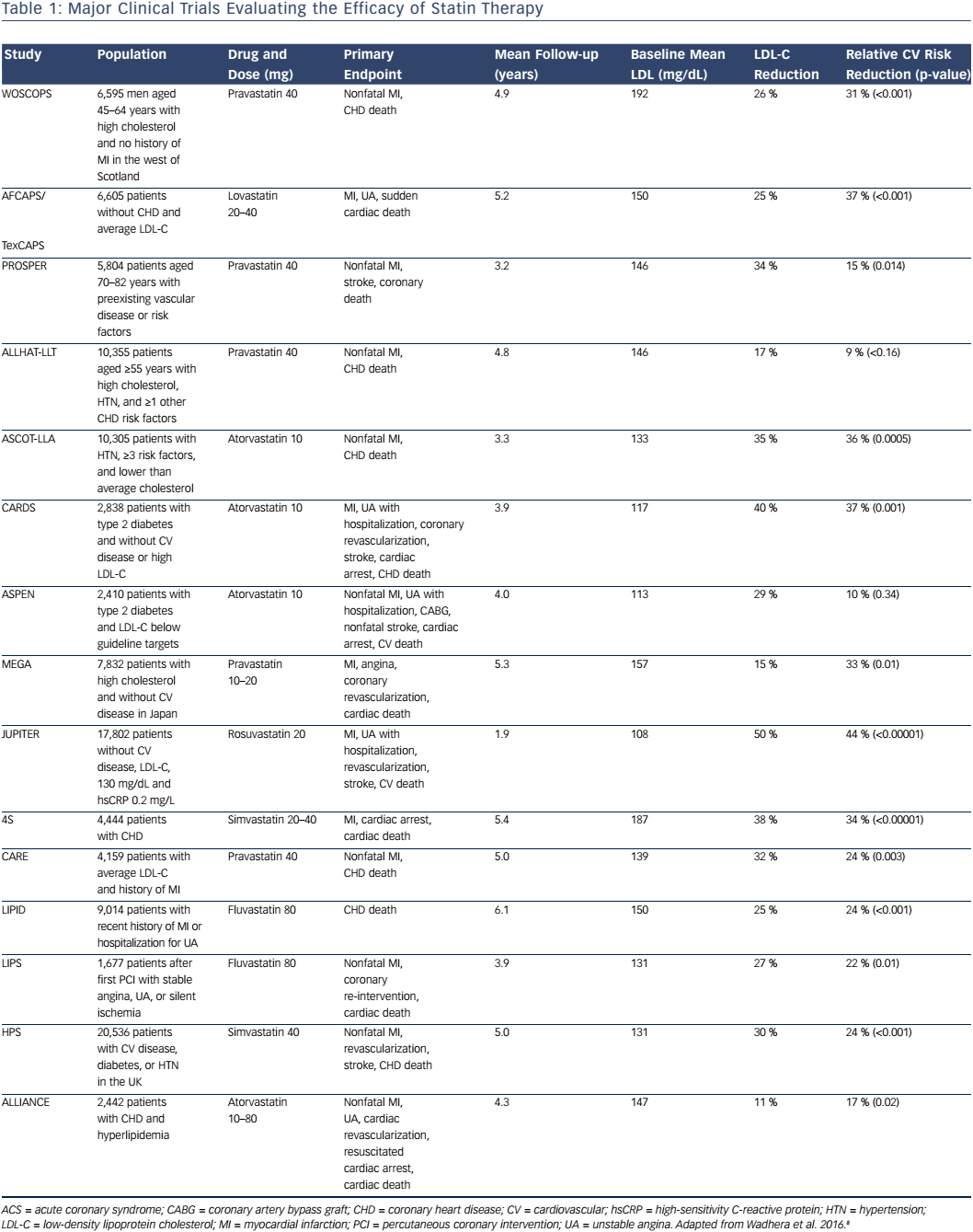
Statins are the cornerstone of treatment for elevated LDL-C levels and are the most commonly prescribed pharmacological agent used to lower LDL-C. Statin medications inhibit hydroxymethylglutaryl CoA reductase, the rate-limiting enzyme in the production of cholesterol, leading to a reduction in intrahepatic cholesterol, up-regulation of hepatic LDL receptors, and enhanced hepatic LDL uptake, thereby lowering serum LDL. Many studies have evaluated the efficacy of statins in the primary and secondary prevention of cardiovascular disease (Table 1).10–21 One meta-analysis of statin trials for primary prevention in low-risk patients with baseline LDL-C levels of 100–160 mg/dL found that with the use of statins, a 39 mg/dL reduction in LDL-C was associated with a 38 % relative risk reduction in nonfatal MI, coronary revascularization, stroke, or coronary death, as well as a 10 % relative risk reduction in all-cause mortality.21 Furthermore, high-intensity statin therapies, defined as those associated with a ≥50 % LDL-C reduction, were shown to be associated with further reductions in LDL-C and an increase in the relative risk reduction of nonfatal MI, coronary revascularization, stroke, or coronary death of approximately 15 %.21
Patients with diabetes mellitus are at increased risk for cardiovascular disease compared to patients without diabetes mellitus; specifically, diabetes mellitus in the absence of prior MI portends a similar risk for coronary heart disease as prior MI without diabetes mellitus.22–24 Furthermore, patients with diabetes mellitus experience a reduction in cardiovascular events with statin therapy similar to that in patients with known coronary heart disease without diabetes.10,12 For these reasons, diabetes mellitus is considered equivalent to coronary heart disease with respect to cardiovascular risk and guidelines for statin therapy, as discussed below.
Despite the well-documented benefits from statins, patient adherence to therapy is frequently challenged by adverse effects. The most commonly reported adverse effect with statins is myalgia; however, the incidence of myalgia attributed to statins is often overestimated based on prior observational data, and placebo-controlled studies have shown nearly identical rates of myalgia in statin and placebo groups.25–28 More serious effects, such as rhabdomyolysis, occur far less commonly, with an incidence of approximately 0.04 %.29 Reversible transaminitis occurs in approximately 0.4 % of patients.29 Importantly, there is also a small and dose-dependent increase in the risk of new-onset diabetes associated with statins.30 The risk appears to be approximately 9 % and increases with higher doses.31
Non-statin Therapies
In addition to statins, there are several other pharmacological therapies that have been studied for the management of dyslipidemia. Cholestyramine, a bile acid sequestrant, was the first medication studied that demonstrated the ability to significantly reduce LDL-C levels, with an approximately 12 % LDL-C reduction and 19 % relative risk reduction in MI or cardiovascular death when compared with placebo.32 Due to the adverse effects of constipation and gastrointestinal upset, cholestyramine is reserved for those with statin intolerance or with inadequate response to stain therapy. The potential additive benefit of reducing LDL-C and cardiovascular events with the concomitant use of statins and cholestyramine has not been prospectively evaluated.
Niacin (nicotinic acid) reduces LDL-C levels by inhibiting the hepatic production of very-low-density lipoprotein cholesterol (VLDL-C) and raises high-density lipoprotein cholesterol (HDL-C) levels by reducing the clearance and transfer of lipids to VLDL-C. Prior to the use of statins, niacin was evaluated against placebo and was found to be effective at lowering total cholesterol and reducing the incidence of nonfatal MI and possibly mortality.33 Despite this, clinical trials evaluating the addition of niacin to statin therapy in patients with cardiovascular disease found no additional clinical benefit, but an increase in adverse events.34,35
Ezetimibe reduces dietary and biliary cholesterol absorption by inhibiting the intestinal and hepatic Niemann-Pick C1-Like 1 protein, thereby lowering total cholesterol and LDL-C levels. Early studies comparing ezetimibe with placebo in the absence of statins found that ezetimibe reduces LDL-C by approximately 15–20 %.36,37 A recent large clinical trial evaluating the addition of ezetimibe to statins in patients with prior acute coronary syndrome found an approximately 24 % reduction in LDL-C levels and a 6.4 % reduction in the relative risk of cardiovascular death, major coronary events, or nonfatal stroke at 7 years.38
PCSK9 Inhibitors
Proprotein convertase subtilisin/kexin type 9 (PCSK9) has been the focus of recent research into the reduction of LDL-C. PCSK9 acts by degrading LDL receptors, thereby reducing the hepatic uptake of LDL.39 Inhibition of PCSK9 leads to decreased LDL receptor breakdown, increased hepatic uptake of LDL, and lower serum LDL levels.40 Studies have shown that loss-of-function mutations of PCSK9 are associated with lower LDL-C levels and a reduction in cardiovascular risk.39 In addition, PCSK9 appears to promote inflammation and endothelial dysfunction, leading to accelerated atherosclerosis, suggesting that PCSK9 inhibition may improve cardiovascular outcomes through mechanisms other than LDL-C reduction alone.41
The findings from trials of two PCSK9 inhibitors have recently been published. A placebo-controlled trial utilizing alirocumab in high-risk patients on statin therapy was found to significantly reduce LDL-C levels by 62 %, with a 48 % reduction in major cardiovascular events.42,43 Evolocumab was also studied against placebo and showed similar results; there was a 61 % reduction in LDL-C and 53 % reduction in major cardiovascular events.44,45 Further large, multicenter clinical trials evaluating both alirocumab and evolocumab are ongoing.42,46 Both PCSK9 inhibitors have been associated with a similar overall rate of adverse events compared to placebo.47 The US Food and Drug Administration has approved both PCSK9 inhibitors as an adjunct to diet and maximally-tolerated statin therapy in adults with atherosclerotic cardiovascular disease or familial hypercholesterolemia who require additional LDL-C lowering.
Novel Agents
In addition to PSCK9 inhibitors, there are several other novel therapies for LDL reduction currently being investigated. One pharmacological therapy targets cholesterylester transfer protein (CETP), which normally works to facilitate the transfer of cholesteryl esters and triglycerides from HDL to lipoproteins. CETP inhibition has been shown to lead to increases in HDL and decreases in LDL-C and lipoprotein(a) levels.8 Early studies of CETP inhibitors have failed to show any clinical benefit, while one study of the CETP inhibitor anacetrapib is currently ongoing.48
Major Guidelines
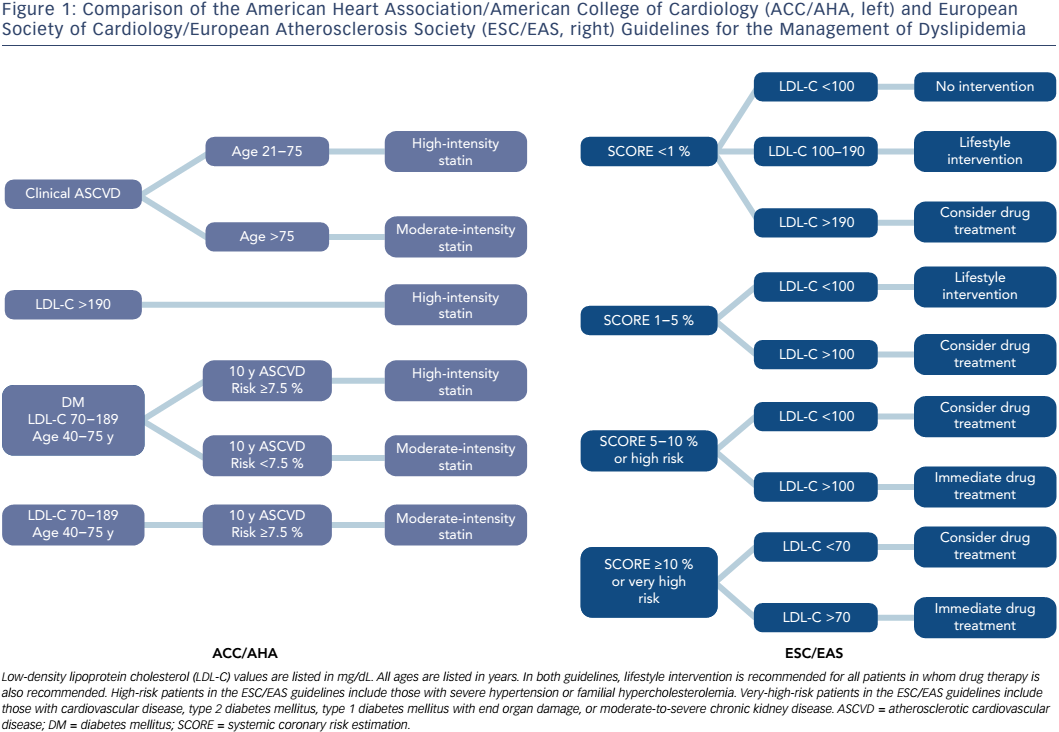
Guidelines for the management of dyslipidemia have been published by multiple different national and international medical societies, including joint guidelines from the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS), and from the American College of Cardiology (ACC) and the American Heart Association (AHA) (Figure 1).49 The European guidelines divide patients into four categories based on risk factors and utilize systemic coronary risk estimation (SCORE) at 10 years in order to establish LDL-C goals for the initiation of pharmacological therapy. In low-risk patients with a SCORE <1 %, the recommended LDL-C goal is <100 mg/dL, but pharmacological therapy is only recommended if LDL-C remains >190 mg/dL despite lifestyle interventions. Moderate-risk patients are defined as those with a SCORE of 1–5 %, and in these patients pharmacological therapy should be considered if LDL-C is >100 mg/dL despite lifestyle modification. High-risk patients are those with a SCORE of 5–10 %, or those with certain significant risk factors, such as familial hypercholesterolemia or severe hypertension. In these patients, the guidelines recommend pharmacological therapy be added to lifestyle modifications for all patients with LDL-C >100 mg/dL, and consideration of pharmacological therapy in patients with LDL-C <100 mg/dL. Very-high-risk patients are defined as those with documented cardiovascular disease, type 2 diabetes mellitus, type 1 diabetes mellitus with end organ damage, moderate to severe chronic kidney disease, or a SCORE >10 %. In these patients, in addition to lifestyle modification, pharmacological therapy is recommended for all patients with LDL-C >70 mg/dL, and should be considered even for those with LDL-C below this level.
The ACC/AHA guidelines were last updated in 2013, with several notable differences when compared to their prior iteration and to the 2011 ESC/ EAS guidelines.50 These guidelines focus on a fixed-dose approach to cholesterol-lowering treatment, in which statin therapy is no longer titrated to achieve LDL goals. This update also introduced a new Pooled Cohort Equation, which incorporates age, sex, smoking, blood pressure, total cholesterol, renal function, and the presence or absence of diabetes, left ventricular hypertrophy, and prior MI or stroke, in calculating an estimated risk of developing atherosclerotic cardiovascular disease (ASCVD) at 10 years. The guidelines recommend fixed-dose, high-intensity statin therapy that results in a >50 % reduction in LDL-C for three broad groups of patients: 1) documented ASCVD between the ages of 21 and 75 years; 2) LDL-C >190 mg/dL and age ≥21 years; and 3) LDL-C 70–189 mg/dL, age 40–75 years, diabetes mellitus, and 10-year ASCVD risk ≥7.5 %. Moderate-intensity statin therapy with a 30–50 % reduction in LDL-C is recommended for the following groups of patients: 1) documented ASCVD and age >75 years; 2) LDL-C 70–189 mg/dL, age 40–75 years, diabetes mellitus, and 10-year ASCVD risk <7.5 %; and 3) LDL-C 70–189 mg/dL, age 40–75 years, and 10-year ASCVD risk ≥7.5 %. Similar to the ESC/EAS guidelines, the ACC/AHA recommends lifestyle modification strategies for all patients and selective use of non-statin pharmacological therapies as adjuncts to statin therapy.
The updated 2013 ACC/AHA guidelines contained two major changes from the previous iterations. The first is the abandonment of the previously recommended strategy to titrate statin dosing to achieve LDL-C goals. Dyslipidemia therapy, based on patients’ risk profiles, with fixed, high- or moderate-intensity statins is more consistent with the majority of clinical trials, which tested the efficacy of statin therapy by using fixed doses. One potential major advantage to this strategy is the avoidance of statin underdosing and undertreatment of LDL-C, which may be more likely to occur when clinicians are encouraged to reduce statin dosing if and when a target LDL-C is reached.51 The ACC/AHA guidelines also now recommend less frequent routine LDL-C monitoring, however, which may create more difficulty in identifying adherence success, and leaves a greater degree of uncertainty regarding if and when to add non-statin therapies to improve LDL-C reduction.52 The second notable change is the introduction of the new Pooled Cohort Equation for the estimation of 10-year ASCVD risk. This risk estimator provides a lower threshold for initiating therapy for primary prevention when compared to the prior guidelines. The 7.5 % 10-year threshold for therapy, which carries a recommendation for a fixed-dose moderate-intensity statin in patients aged 40–75 years with LDL-C of 70–189 mg/dL, corresponds to a European SCORE of 2.5 %, at which drug therapy can be considered at LDL-C >100 mg/dL, but is not strictly recommend.53
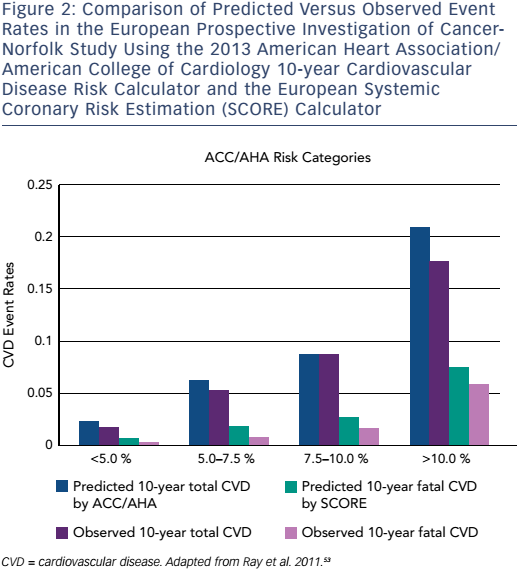
This paradigm change in the approach to primary prevention and the recommendation to treat a significantly greater number of patients has garnered a considerable amount of publicity and criticism. Some recent studies have suggested that this strategy is a cost-effective method to improve population health.53–56 One study evaluated the European Prospective Investigation of Cancer (EPIC)-Norfolk population in order to assess the impact of the ACC/AHA guidelines on population health.53 The authors found that the new ACC/AHA guidelines would result in up to 65 % more individuals being treated with statins for primary prevention and would mildly overestimate the incidence of ASCVD over 10 years (Figure 2). The authors found no significant benefit of the ACC/AHA Pooled Cohort Equation over SCORE in the EPICNorfolk population.
Overall, the ACC/AHA guidelines recommend treating an increased number of individuals for primary prevention and recommend treating all patients with higher statin doses, while the ESC/EAS guidelines take a less conservative approach to primary prevention, and recommend generally lower statin doses, titrated to LDL-C levels. The ACC/AHA risk calculator has not been prospectively evaluated and appears to overestimate cardiovascular risk, particularly in some ethnic populations.
Our Approach to Patient Selection
Given the major differences between the AHA/ACC and ESC/EAS guidelines, clinicians today are faced with challenges regarding choosing appropriate individuals to treat with statins for the primary prevention of cardiovascular disease. Adopting the AHA/ACC guidelines will result in a significant increase in the number of individuals treated and will significantly reduce average LDL-C levels. However, the long-term cost of this approach is not currently known. Given the burden of cardiovascular disease and enormous success at reducing cardiovascular morbidity and mortality over the past few decades with LDL-lowering therapies, we favor considering a risk-based assessment of patients including the fundamental basis of the 2013 AHA/ACC guidelines, and an individualized patient-based discussion regarding therapy. We view these guidelines as a framework for when to consider therapy, identify patients in whom earlier initiation of statin therapy may be beneficial, and identify those who may benefit from higher doses of statins, such as high-risk diabetics. In patients without known diabetes mellitus or ASCVD who have borderline 10-year risk scores that would warrant therapy, however, we favor an extensive risk and benefit discussion in order to make a joint decision about long-term therapy, taking into account patients’ preferences and values in order to ensure compliance and potential benefit from lipid-lowering therapy.
Conclusion
Cardiovascular disease continues to be the number one cause of morbidity and mortality in the United States and worldwide, with treatment of dyslipidemia being the most effective modifiable target for improving cardiovascular outcomes. Statins are the cornerstone of LDLC-lowering therapy, while PCSK-9 inhibitors are now available for addition to maximally-tolerated statin therapy in the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease, who require additional lowering of LDL-C. The most recent update to the ACC/AHA guidelines recommends treating a greater number of patients for primary prevention and aiming to achieve percentage-based LDL reduction rather than specific LDL levels. The accuracy of the new ACC/AHA risk calculator and the long-term costs of this approach are not currently known. Moving forward, it will be helpful to prospectively validate the Pooled Cohort Equation’s accuracy at predicting cardiovascular risk and to test the results across various ethnic and geographic populations. For now, these new guidelines serve as a framework within which patients can be evaluated, while individual treatment decisions should always involve an individualized patient-centered approach, with consideration of individual risks, benefits, and values in choosing the most appropriate treatment strategy.