Prevalence of Cardiovascular Disease
CVD is the leading cause of death in Europe and the United States. CVD mortality is on the decline in many countries, and in several European countries, cancer has now surpassed CVD as the leading cause of death.1 However, CVD remains the leading cause of death for both men and women in the United States and results in more female deaths than cancer, lung disease and diabetes combined. Until recently, the annual mortality rate for CVD in the United States was higher among women compared with men. However, the latest statistics show a dramatic change. In 2013, for the first time since 1984, more men died of cardiovascular disease than women.2 As a result of this historical gender disparity, significant efforts have been focused on increasing public awareness of the burden of CVD in women. A national survey conducted in 2012 showed that 51 % of women were aware that CVD was the leading cause of death in women, compared with 30 % in 1997.3
Symptoms of Coronary Heart Disease
Women present with coronary heart disease (CHD) on average 10 years later than men. Men are more likely to present with a myocardial infarction (MI) as their initial manifestation of CHD, while women are more likely to present with angina. The most common symptom of CHD in both women and men is chest pain. Other typical anginal equivalents include dyspnea and diaphoresis. Although chest pain is the most common symptom, women are more likely to have atypical symptoms of CHD including jaw, back or arm pain, nausea and fatigue.4,5 It is important that clinicians and the general public are aware of these atypical symptoms to ensure prompt evaluation and treatment in symptomatic women. Clinical misdiagnoses and delays in delivery of evidence-based, lifesaving cardiovascular care can occur as a result of this lack of awareness.
Risk Factors for CHD
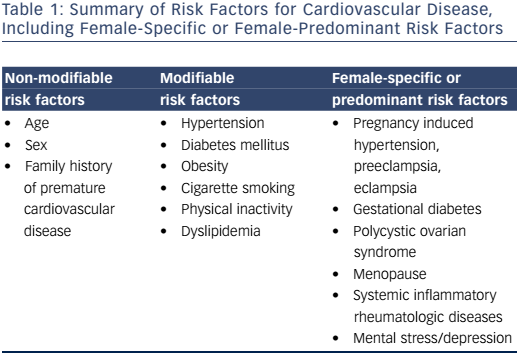
Traditional risk factors for CVD are well known, and include diabetes mellitus, age, family history of premature coronary artery disease (CAD), tobacco use, hypertension, obesity and physical inactivity (Table 1). Some of these factors impact a woman’s risk of CVD to a greater degree than they do in men. Women with diabetes are 3–7 times more likely to develop CAD. Men with diabetes, on the other hand, have a 2–3-fold increased risk for CAD.6 Among young and middle-aged women – a group that generally has lower rates of CAD than their male counterparts – the presence of diabetes is associated with a 4–5-fold higher rate of CAD.7 Tobacco use is associated with a higher risk of MI in women compared with men.8
Hypertension is one of the most common modifiable risk factors for CVD. It is more prevalent in men than women until age 45. After age 55, women have a higher prevalence than men.2,9 Some have attributed this to women living longer than men and as a result, the elderly population of women is larger than their male counterparts. Regardless of this, women with hypertension are less likely to be treated to goal.10
Several hormone-related factors can result in hypertension in women. Oral contraceptives (OC) will increase blood pressure slightly in most women, but rarely result in malignant hypertension. Different OCs have variable effects on blood pressure. For example, in the Nurses’Health Study, oral contraceptives with low doses of estrogen increased the risk of hypertension.11 Prospective studies have shown that discontinuation of OC results in blood pressure returning to baseline within a few months.12
Despite studies demonstrating equal lipid-lowering benefit in men and women, women are less likely to be treated with statins after an MI.13,14 Furthermore, menopause has specific effects on lipoproteins levels. After menopause, triglyceride and low-density lipoprotein cholesterol levels rise and high-density lipoprotein cholesterol levels decline, resulting in an unfavorable lipid profile. It is uncertain whether these changes occur as a result of hormonal or lifestyle changes.
Female-specific or Female-predominant Risk Factors
There are also additional female-specific or female-predominant risk factors that clinicians and patients must be aware of and include in the assessment of CVD in women. The association between systemic autoimmune inflammatory disease and increased cardiovascular risk is well known. Although the mechanism is not well understood, there is extensive literature demonstrating that systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) confer an increased risk of CAD independent of traditional risk factors alone.15–18 Accelerated atherosclerosis is a well-recognized finding in both groups.15,19,20 Both SLE and RA occur at higher rates among women than in men. Premature cardiovascular mortality has been seen in patients with SLE with a mean age of MI at age 52 in one cohort.21 CVD is the leading cause of morbidity and mortality in patients with SLE. Patients with RA have an increased risk of CHD, and there is a 2-fold greater risk of developing heart failure and a 1.5–2-fold increased risk of CAD compared with the general population. They are also less likely to have angina as the presenting symptom for CHD.
Gestational diabetes mellitus (GDM) is associated with increased future risk of type 2 diabetes mellitus and CVD compared with women without GDM.22–24 Pregnancy-induced hypertension is a spectrum of disorders that includes preeclampsia-eclampsia, chronic hypertension, chronic hypertension with superimposed preeclampsia and gestational hypertension.25 New-onset gestational hypertension, preeclampsia and eclampsia have been shown to be associated with increased future cardiovascular risk and cardiovascular risk factors.26–28 A metaanalysis reported the relative risk of CHD in women with a history of preeclampsia to be more than double that of women who have not had preeclampsia.29 Women who deliver preterm or a small for gestational age baby are also at increased future risk for CHD,30–32 and women who suffer from spontaneous pregnancy loss are at increased future risk for coronary heart disease and MI. A history of stillbirth was associated with a >3.5 times higher risk of MI (age-adjusted hazard ratio [HR] 3.70; (95 % CI [1.69–8.11]). Recurrent miscarriage (more than three occurrences) was associated with an approximately 9 times higher risk of MI (ageadjusted HR 8.90; 95 % CI [3.18–24.90]).33 In a meta-analysis of 10 studies, miscarriage was associated with a higher likelihood of developing future CHD with an odds ratio (OR) of 1.45 (95 % CI [1.18–1.78]).34
The risk for CAD in women increases beginning 10 years from the onset of menopause. Women who undergo premature menopause, as a result of radiation, chemotherapy or surgery, are at increased risk for CAD compared with those who transition into natural menopause 10 years later. However, the findings of the Women’s Health Initiative and the Heart and Estrogen/Progestin Replacement Study provided conclusive evidence that menopausal hormone therapy is not beneficial in preventing heart disease.35,36 As a result, it is not recommended for the primary or secondary prevention of CVD
Psychosocial factors have also been associated with CHD.37 According to 2014 statistics, 15.7 million (6.7 %) adults in the United States had at least one depressive episode within the prior year.38 More women have depression compared with men in every age group, with rates of depression among women nearly twice those of men.39,40 The prevalence of depression is much higher in the cardiac population at nearly 15 %, which is three times that seen in the general population.41 Long-term prospective studies have found depression to be associated with the development of CHD, independent of other risk factors for CHD.42-44 Data support a correlation between severity of depression and the risk of cardiovascular events.45 In a prospective study among women without known CVD, symptoms of depression were directly associated with risk of CHD events in age-adjusted and multivariate models.46
In up to two-thirds of patients with MI, mild depressive symptoms occur post-MI,47 and major depressive disorder has been reported to develop in almost 20 % of patients after an MI.48,49 Anxiety has also been associated with increased risk of fatal CHD in women.50 Unlike traditional risk factors for CAD, there are no current cardiology guidelines for the treatment of psychosocial risk factors. However, given the robust literature demonstrating an association between depression and cardiovascular events and poor outcome post-MI, it is imperative that patients with CAD or recent MI are screened and treated for depression. The 2011 American Heart Association/American College of Cardiology Foundation recommends healthcare providers consider the treatment of depression for its other clinical benefits, as treatment has not been shown to improve cardiovascular outcomes.51
Polycystic ovarian syndrome (PCOS) is the most common endocrine disorder in women of reproductive age. Women with PCOS are at increased risk for insulin resistance, type 2 diabetes mellitus, dyslipidemia and the development of metabolic syndrome.52–54 A study by Christian etal showed coronary artery calcification to be more prevalent in women with PCOS compared with controls (39 % vs. 21 %; OR 2.4; p=0.05).55 Given the increased prevalence of cardiovascular risk factors among women with PCOS, aggressive risk factor screening, modification, and treatment should be pursued in this group.
Risk Assessment Models
Several risk assessment calculators have been developed for the estimation of CHD risk. The Framingham Risk Score (FRS) was first published in 1998 and provides a 10-year estimate of CHD risk.56 The 2008 revised FRS was modified to include additional cardiovascular clinical endpoints including heart failure, transient ischemic attack and symptomatic peripheral arterial disease. While this risk model provided an improved estimate of CVD, it still suffers from some challenges, and underestimates risk in women.
One of the limitations of the FRS is that it does not account for the increased cardiovascular risk in patients with systemic inflammatory diseases. Furthermore, more women are affected by SLE and RA, and therefore their risk of CVD may be underestimated using this risk model. The FRS provides a short-term (10-year) risk estimate of CHD in women. Women traditionally tend to have lower short-term cardiovascular risk, but a higher lifetime cardiovascular risk. Finally, it does not include family history, pregnancy-related risk attributes, or evidence of subclinical CVD in cardiovascular risk estimation.
Other risk prediction models for women include the Reynolds Risk Score). Key differences between the Reynolds Risk Score and other cardiovascular prediction models are the inclusion of high-sensitivity C-reactive protein, and family history of MI.57 The use of this scoring system may be more accurate at predicting risk for CVD in women with chronic inflammatory diseases.
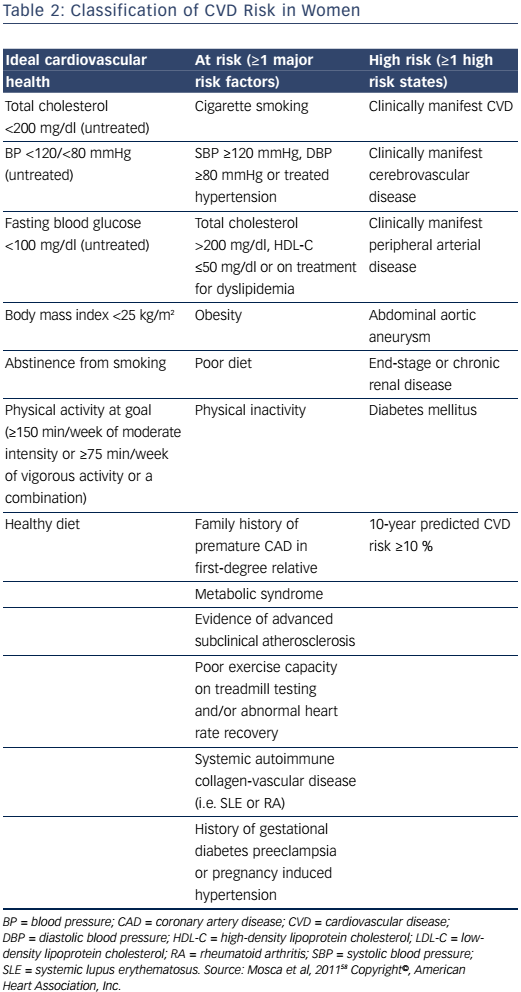
The 2011 Effectiveness-Based Guidelines for the Prevention of CAD in Women outlines an algorithm for risk stratifying women.58 It provides an algorithm for the classification of women as ideal cardiovascular health, at-risk, or high-risk for CVD, based on several criteria (Table 2). Those classified as at risk or high risk are appropriate candidates for aggressive cardiovascular risk-factor modification and secondary preventive efforts to reduce recurrent atherosclerotic cardiovascular events. For women who are considered to have ideal cardiovascular health, initiatives focused on lifestyle modification to prevent the development of clinical risk factors for CVD or clinical atherosclerotic CVD are imperative. Given the high lifetime risk for CVD in women, these preventive strategies are of utmost importance for women who do not have clinical atherosclerotic CVD. However, this document predates the 2013 American College of Cardiology and American Heart Association (ACC/AHA) prevention guidelines.
The ACC and AHA developed a new calculator in 2013 for the estimation of cardiovascular events.59 The pooled cohort equation is gender-specific provides both a 10-year atherosclerotic cardiovascular disease risk, and a lifetime risk for CVD (http://tools.acc.org/ASCVDRisk-Estimator). This is significant given the higher lifetime risk for CVD in women and allows providers to recommend aggressive lifestyle changes and medications to reduce cardiovascular risk and events.
Despite the advantages and disadvantages of each of the multivariate risk models, no single risk model is the most accurate at estimating cardiovascular risk. The best method of estimating cardiovascular risk in asymptomatic individuals involves using one of the multivariate risk models combined with laboratory results, taking into account of family history of premature CAD and the presence of non-traditional risk factors.
Evaluation of Symptomatic Women for CHD
The exercise treadmill test (ETT) is the oldest and most commonly used test for evaluating CAD in symptomatic women. In women who have a normal baseline electrocardiogram (ECG) and good functional capacity, the ETT is the appropriate test in the initial evaluation of women with suspected CHD. It is recommended by the ACC/AHA as the initial diagnostic test of choice in symptomatic women with intermediate pretest likelihood of CAD.60
For clinicians who are unsure of a woman’s functional capacity, the Duke Activity Status Index is helpful. It is a 12-item questionnaire that can be used to estimate metabolic equivalents of task (METs) associated with activities of daily living. The information provided identifies those who are unable to achieve 5 METs and should be considered for pharmacologic stress testing.61 The sensitivity and specificity of detecting obstructive CAD in women using the ETT is lower than in men.62 This is because of the lower exercise capacity and prevalence of obstructive CAD, as well as the high prevalence of ST segment depression during exercise in women compared with men. However, the ETT is not a futile test. It can also identify myocardial ischemia, which may be unrelated to obstructive coronary disease such as in coronary microvascular dysfunction. The Duke Treadmill Score and functional capacity provide valuable prognostic information in the risk stratification of symptomatic women.63–65 A low Duke Treadmill Score is associated with <1 % annual mortality rate compared with an annual mortality rate of nearly 5 % in those with a high Duke Treadmill Score .63 Those who have lower functional capacity have higher cardiovascular event rates than those who are able to achieve at least 5 METs on exercise testing. A negative stress ECG also has a high negative predictive value.
Stress echocardiogram is performed with the combination of echocardiogram and either exercise or the use of a pharmacologic agent. This modality is appropriate for intermediate-risk symptomatic women who have poor functional capacity, an abnormal baseline ECG that precludes interpretation of the ST segment during exercise, or an intermediate Duke Treadmill Score. The addition of imaging to an ETT improves the diagnostic accuracy of detecting obstructive CAD above the ETT alone. The sensitivity and specificity of the ETT for detecting obstructive CAD increases from 31–71 % and 66–86 %, respectively, to 80–88 % and 81–86 %, respectively, with the addition of echocardiography.66–69 In addition to wall motion abnormalities, the stress echocardiogram can identify other causes of chest pain or dyspnea in women including hypertrophic cardiomyopathy, valvular heart disease, or pulmonary hypertension.
Stress single photon emission computed tomography myocardial perfusion imaging (SPECT) can be used in the diagnostic evaluation of intermediate-risk symptomatic women who have limited functional capacity or an abnormal baseline ECG. Pharmacologic stress SPECT has a diagnostic sensitivity of 91 % and specificity of 86 % in women. Stress SPECT myocardial perfusion imaging provides valuable prognostic information based on the size and extent of ischemic perfusion defects, as well as ejection fraction. Cardiovascular events are lower in women with small reversible defects, confined to single coronary artery territory or with preserved left ventricular ejection fraction compared with those who have multiple or large perfusion defects or left ventricular ejection fraction <35 %.
Stress photon emission tomography (PET) has been useful in improving the diagnostic accuracy of detecting obstructive CAD in women who have breast attenuation artifact on stress SPECT imaging or poor windows on stress echocardiography. Unlike stress echocardiography or stress SPECT imaging, exercise cannot be performed during stress PET testing because of the short half-life of rubidium. It is an appropriate diagnostic test for detecting obstructive CAD in women who are of intermediate pretest probability for CAD, but are unable to exercise or have attenuation artifacts on SPECT imaging.
Stress cardiac magnetic resonance imaging (CMR) has become more attractive in the assessment of symptomatic women. It has the advantage of avoiding the use of radiation during stress testing compared with stress PET or SPECT. This is an issue that deserves consideration when determining which stress test to perform in a young, premenopausal woman who cannot exercise. In addition, CMR provides additional information pertaining to cardiac morphology, coronary anatomy and evaluation of the thoracic aorta in women presenting with chest pain.
Conclusion
Cardiovascular risk assessment in women involves a thorough history, physical examination, and laboratory testing for the identification of risk factors for CVD. Risk assessment calculators can be used to stratify an asymptomatic woman into specific risk categories and further direct preventive strategies to reduce the future risk of CVD. Asymptomatic women who have risk factors for CVD should undergo aggressive modification of cardiovascular risk factors. Symptomatic women should undergo appropriate testing to evaluate for obstructive and nonobstructive CAD.