Diabetes mellitus is one of the most common chronic diseases, affecting >30 million people in the US and 422 million worldwide.1,2 Alarmingly, both the incidence and prevalence of type 2 diabetes have doubled in the US since 1990.3 This is driven by an aging population, obesity, physical inactivity, and prolonged survival in those with diabetes, among other factors. It is estimated that diabetes will affect >54 million people in the US by 2030.4 Cardiologists routinely care for patients with diabetes, as those with the condition have a two- to four-fold increased risk of developing coronary heart disease.5 Indeed, coronary heart disease is the leading cause of morbidity and mortality in those with diabetes, with over one-third having a myocardial infarction in their lifetimes.6 Furthermore, diabetes patients with an acute coronary syndrome have worse clinical outcomes compared with people without diabetes.7 Patients with diabetes also have a two- to five-fold increased risk of developing heart failure.8
The UK Prospective Diabetes Study, among others, found that intensive glycemic control significantly reduces microvascular complications but fails to modify macrovascular risk in patients with diabetes.9 While contemporary meta-analyses suggest that intensive glycemic control does reduce the risk of cardiovascular events, these benefits appear modest compared with the cardiovascular risk reductions associated with the modification of other traditional risk factors.10,11 Importantly, several classes of diabetes medications have been associated with adverse cardiovascular events. Specifically, sulfonylureas are associated with increased cardiovascular mortality while thiazolidinediones and dipeptidyl peptidase-4 (DPP-4) inhibitors are associated with higher rates of heart failure.
Sulfonylureas have been used for the treatment of type 2 diabetes for >50 years. Their main effect is through raising serum insulin concentrations via stimulation of the pancreatic beta cells.12 Concerns over the cardiovascular safety of these drugs arose following the University Group Diabetes Program study in 1975, which demonstrated excess cardiac deaths in patients treated with tolbutamide compared with placebo or insulin.13 Subsequent studies with other agents in this drug class demonstrated similar results.14–16 For example, a recent review and meta-analysis of 82 randomized controlled trials and 26 observational studies showed an increased risk of all-cause mortality, cardiovascular-related mortality, myocardial infarction, and stroke with sulfonylureas compared with other glucose-lowering drugs.17 As a result of the totality of evidence, all drugs in the sulfonylurea class carry a ‘black box’ warning for increased risk of cardiovascular mortality.
Thiazolidinediones are synthetic ligands for peroxisome proliferative-activated receptor gamma, which improves insulin sensitivity in peripheral tissues.18 First approved by the US Food and Drug Administration (FDA) in 1999, the safety of these agents was called into question following a 2007 meta-analysis showing that rosiglitazone was associated with an increased risk of myocardial infarction and death from cardiovascular causes.19 These drugs have also been associated with an increased risk of heart failure.20,21 More recent studies dispute the increased risk of myocardial infarction with rosiglitazone and there are data supporting the cardioprotective effects of pioglitazone.22,23 However, the increased risk of heart failure, weight gain and bone fractures, along with a potential association with bladder cancer, has led to significant reductions in the use of these drugs.24 The concerns about peripheral edema and heart failure have also resulted in product labels cautioning against the use of thiazolidinediones in patients with heart failure, with a contraindication for initiation in patients with New York Heart Association Class III or IV.25
DPP-4 inhibitors are a class of oral hypoglycemic agents that block the action of endogenous DPP-4 – a protease that degrades incretin hormones such as glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide, thereby increasing their serum concentrations. GLP-1 and gastric inhibitory polypeptide are released in the postprandial state and act by reducing gastric motility, stimulating insulin secretion, and decreasing postprandial glucagon release.26 The initial cardiovascular safety and efficacy trial for saxagliptin showed no effect on ischemic events but an increased rate of heart failure hospitalizations.27 Another agent in the class, alogliptin, was associated with a higher, but not statistically significant, risk of heart failure hospitalizations.28 Two subsequent meta-analyses found that DDP-4 inhibitors were associated with an increased risk of heart failure.29,30 Despite the above data, the association with heart failure does not appear to be a class effect, as a double-blind, placebo-controlled trial of sitagliptin versus placebo among patients with type 2 diabetes and atherosclerotic vascular disease found no affect on the risk of heart failure hospitalizations.31
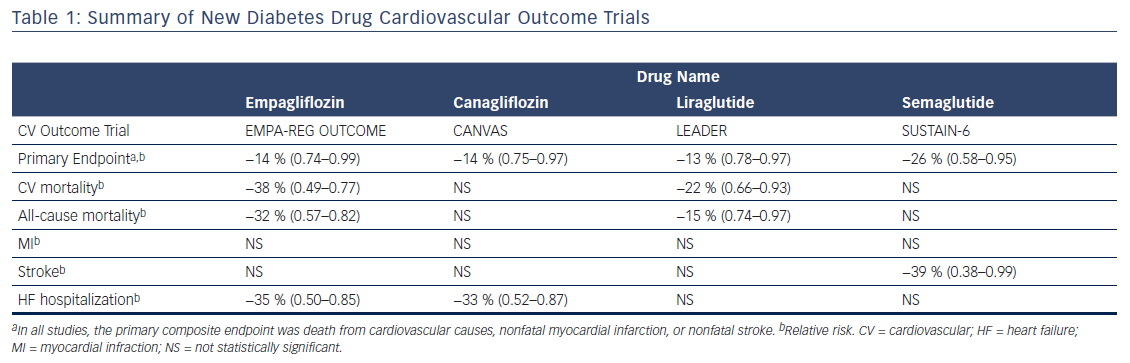
Due to safety concerns with some glycemic agents, in 2008 the FDA along with the European Medicines Agency mandated that new type 2 diabetes drugs demonstrate cardiovascular safety through large-scale randomized controlled trials.32 In the years since their inception, not only have these paradigm-shifting regulations led to diabetes drug trials that demonstrate cardiovascular safety, but several have recently shown improved cardiovascular outcomes, including reduced cardiovascular and all-cause mortality. Currently, there are two classes of diabetes medications with demonstrated reductions in cardiovascular and all-cause mortality: sodium–glucose cotransporter-2 (SGLT-2) inhibitors and GLP-1 analogues (see Table 1).
Review of Clinical Trial Evidence
SGLT-2 Inhibitors
SGLT-2 is a glucose transporter in the proximal tubule of nephrons that is responsible for 90 % of glucose reabsorption.33 Individuals with loss-of-function mutations in the gene for SGLT-2 rarely develop type 2 diabetes or obesity, an observation that led to the development of pharmaceuticals to target this protein pathway.34 Inhibitors of SGLT-2 block glucose reabsorption in the nephron, but also cause decreased sodium reabsorption. While the primary effect of these agents is on serum glucose reduction, they also promote a modest diuretic effect, weight loss, and lower blood pressure.35,36
The cardiovascular outcomes trial for the SGLT-2 inhibitor empagliflozin was the first study of a new diabetes drug to demonstrate a statistically significant reduction in cardiovascular events. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) randomized 7,020 patients with type 2 diabetes and established cardiovascular disease to 10 mg or 25 mg of empagliflozin or placebo once daily.37 Patients in both groups received additional off-study treatments for glycemic control at the discretion of their providers. The primary composite outcome was death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. The outcome measures were analyzed using the pooled empagliflozin group. At a median follow up of 3.1 years, patients receiving empagliflozin had a 14 % relative risk reduction in the primary outcome (hazard ratio [HR] 0.86, 95.02 % confidence interval [CI] 0.74–0.99, p=0.04 for superiority). This result was primarily driven by a 38 % relative risk reduction in cardiovascular death (HR 0.62, CI 0.49–0.77, p<0.001). There was no between-group difference in the risk of myocardial infarction or stroke. Patients randomized to empagliflozin also had a 32 % relative reduction in the risk of death from any cause (HR 0.68, CI 0.57–0.82, p<0.001) and a 35 % reduction in heart failure hospitalizations (HR 0.65, CI 0.50–0.85, p=0.002). Those treated with empagliflozin had a mean reduction in weight of approximately 2 kg and a mean reduction in systolic blood pressure of approximately 4 mmHg. Kidney disease outcomes were also investigated as a secondary endpoint in the EMPA-REG OUTCOME trial. There was a significant reduction in incident or worsening nephropathy and lower rates of clinically relevant renal events in patients randomized to empagliflozin.38 There was a similar benefit with each dose of empagliflozin studied in the trial. While the drug was well tolerated overall, there was an increased rate of genitourinary infections among patients receiving empagliflozin compared with placebo.
The safety and efficacy of canagliflozin was studied in the CANaglifozin cardioVascular Assessment Study (CANVAS) Program, which integrated data from two trials of 10,142 patients with type 2 diabetes and high cardiovascular risk who were randomized to receive this SGLT-2 inhibitor or placebo.39 High cardiovascular risk was defined as either symptomatic atherosclerotic cardiovascular disease (65.5 % of participants) or being ≥50 years of age with two or more cardiovascular risk factors. The primary composite outcome was death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. At a mean follow-up of 188 weeks, treatment with canagliflozin led to a 14 % relative risk reduction in the primary endpoint compared with placebo (HR 0.86, 95 % CI 0.75–0.97, p<0.001 for noninferiority, p=0.02 for superiority). Superiority was not demonstrated for the first secondary endpoint (all-cause mortality, p=0.24), so according to the statistical plan further hypothesis testing was discontinued and other results were reported as exploratory and not considered statistically significant. These secondary outcomes included a 33 % relative risk reduction in heart failure hospitalizations in those treated with canagliflozin (HR 0.67, CI 0.52–0.87). Unlike empagliflozin, canagliflozin was not shown to reduce cardiovascular or all-cause mortality. Those treated with canagliflozin had a mean body weight reduction of 1.6 kg and a mean systolic blood pressure reduction of 3.93 mmHg. While it did not reach statistical significance, there was a trend toward benefit with respect to the progression of albuminuria and adverse renal outcomes. Notably, there was a significantly increased risk of amputations in the canagliflozin group (HR 1.97, CI 1.41–2.75) with 71 % of amputations occurring at the level of the toe or metatarsal. Similar to empagliflozin, there was an increased risk of genitourinary infections among patients treated with canagliflozin.
Interestingly, empagliflozin and canagliflozin produced only modest hemoglobin HbA1c reductions (0.54 % and 0.58 %, respectively) with convergence of levels toward the end of the trials. Moreover, the cardiovascular benefits of both drugs were noted early. These observations suggest that their benefits may result from direct cardiovascular effects rather than improved glycemic control. While there were modest improvements in body weight and blood pressure in both studies, these are unlikely to fully account for the cardiovascular benefits demonstrated. There is a general belief that the rapid natriuresis and glycosuria induced by these agents may explain the early cardiovascular benefits through reductions in circulating volume and resultant decreases in filling pressures.40,41 Other postulated mechanisms by which SGLT-2 inhibitors exert their cardiovascular benefits include reduction in glomerular hypertension, red blood cell expansion, and ketone-body elevation.36,37,40,42
As a result of the EMPA-REG OUTCOME trial, in 2016 the FDA approved empagliflozin to reduce the risk of cardiovascular death in patients with type 2 diabetes.43 Canagliflozin does not yet have this FDA-approved indication.
GLP-1 Analogues
GLP-1 is a peptide hormone that increases insulin secretion and decreases glucagon release in a glucose-dependent manner.44 GLP-1 analogues were developed to exploit these effects. They have been found to reduce serum glucose levels and weight through increasing glucose-dependent insulin secretion, decreasing glucagon secretion, delaying gastric emptying, and increasing satiety.44,45 Two drugs from this class, liraglutide and semaglutide, have been shown to improve cardiovascular outcomes.
Liraglutide is an injectable, long-acting GLP-1 receptor analogue that has been shown to lower serum glucose levels, reduce blood pressure, and promote weight loss.44,46 The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial randomized 9,340 patients with type 2 diabetes and high cardiovascular risk to receive liraglutide or placebo.47 High cardiovascular risk was defined as established cardiovascular disease or age >60 years with one or more cardiovascular risk factors. With a median follow-up of 3.8 years, patients treated with liraglutide had a 13 % relative risk reduction in the primary composite outcome of cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke (HR 0.87, 95 % CI 0.78–0.97, p<0.001 for noninferiority, p<0.01 for superiority). This effect was largely driven by a 22 % reduction in cardiovascular death. The rate of all-cause mortality was also significantly reduced in the liraglutide group compared with placebo (HR 0.85, CI 0.74–0.97, p=0.02). Patients treated with liraglutide had a mean weight loss of 2.3 kg and there was no significant difference in blood pressure compared with placebo. A pre-specified secondary analysis showed that liraglutide use resulted in lower rates of the development and progression of diabetic kidney disease.48 This effect was driven primarily by a reduction in new-onset persistent macroalbuminuria. The rates of renal adverse events were similar between the two groups. Finally, there was no significant reduction in heart failure hospitalizations among patients treated with liraglutide compared with placebo (4.7 % versus 5.3 %, p=0.14). As a class, GLP-1 analogues carry a warning for increased incidence of pancreatitis; however, rates were similar with liraglutide compared to placebo in this trial. More patients in the liraglutide group discontinued therapy due to adverse events, which were largely driven by gastrointestinal disorders.
Subsequently, the once-weekly injectable GLP-1 analogue semaglutide was assessed for cardiovascular safety in the Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes (SUSTAIN-6) trial.49 A total of 3,297 patients with type 2 diabetes and high cardiovascular risk, defined in the same way as the LEADER trial, were randomized to semaglutide or placebo. The composite primary endpoint was cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. After a median follow-up of 2.1 years, patients treated with semaglutide had a 26 % relative risk reduction in the primary endpoint (HR 0.74, 95 % CI 0.58–0.95, p<0.001 for noninferiority). This reduction was due to fewer nonfatal strokes (1.6 % versus 2.7 %, p=0.04) and myocardial infarctions (2.9 % versus 3.9 %, p=0.12). Unlike liraglutide, semaglutide was not found to reduce cardiovascular mortality. There was also no significant reduction in heart failure hospitalizations among patients treated with semaglutide. Patients in the treatment group had mean body weight reductions of between 3.6 kg and 4.9 kg based on drug dose. Mean systolic blood pressure was 1.3 mmHg lower in the treatment group than placebo. Like liraglutide, more patients treated with semaglutide discontinued therapy due to adverse events, which were mainly gastrointestinal. However, semaglutide was also associated with a significant increase in rates of retinopathy complications.
Two other GLP-1 receptor agonists, exenatide and lixisenatide, have been assessed for cardiovascular safety. The cardiovascular outcome trials for these drugs showed no increase or decrease in cardiovascular events or adverse events compared with placebo.50,51 A cardiovascular outcome trial was stopped early for the candidate GLP-1 receptor agonist taspoglutide due to reported anaphylactoid and anaphylactic reactions.52
The cardiovascular benefits seen with liraglutide and semaglutide occurred later than in the SGLT-2 inhibitor trials. This suggests that their mechanism of action involves the modification of atherothrombotic pathways, rather than hemodynamic alterations. Other postulated mechanisms include blood pressure reduction, renal protective effects, and weight loss.53
In 2017, based on the results of the LEADER trial, the FDA approved a new indication for liraglutide to reduce the risk of major cardiovascular events in patients with type 2 diabetes and established cardiovascular disease.54 Semaglutide is currently being reviewed by the FDA.
Implications for Clinical Practice
Until recently, most US-based guidelines for type 2 diabetes pharmacotherapy management focused primarily on HbA1c and weight effects as well as risk of hypoglycemia. The results of recent cardiovascular outcome trials have prompted a change in diabetes management guidelines.55 For example, the 2017 American Diabetes Association standards of care recommend considering empagliflozin or liraglutide in patients with suboptimally controlled diabetes and atherosclerotic cardiovascular disease, recognizing the cardiovascular benefits of these agents.56 The Canadian Diabetes Association was the first body to prioritize cardiovascular disease status in choosing add-on therapy in its 2016 interim update, stating “the presence of clinical cardiovascular disease and the effect of antihyperglycemic agents on cardiovascular outcomes should be considered the top priority in choosing add-on treatment regimens.”57 Governing bodies have also started recognizing the cardiovascular effects of these new agents. The 2016 Joint European Society of Cardiology guidelines give a Class IIa, Level B recommendation to consider SGLT-2 inhibitors early in the course of type 2 diabetes in patients with comorbid cardiovascular disease to reduce cardiovascular and total mortality.58 It is likely that more societies and guideline committees will recognize the cardiovascular benefits of these agents and prioritize their use in treatment in the coming years.
Excitement over the cardiovascular outcomes associated with these new agents must be tempered by an objective analysis of their cost-effectiveness compared with alternative treatments. Indeed, the average monthly cost for empagliflozin is $410, as compared with <$10 per month for other agents such as metformin and glipizide.59–61 In the UK, Huseyin Naci and colleagues found that prevention of one cardiovascular event with empagliflozin would cost approximately £73,431 if used in a high-risk population.62 This cost would increase to £322,361 if offered to lower-risk patients.62 Canagliflozin was found to be cost-effective compared with sitagliptin in patients with poorly controlled type 2 diabetes in a Mexican study.63 In a US analysis of liraglutide compared with sitagliptin, the incremental cost-effectiveness ratio was between $25,742 and $37,234 per quality-adjusted life year gained, based on liraglutide dose.64 In all sensitivity analyses, the incremental cost-effectiveness ratios remained below the commonly accepted threshold of $50,000 per quality-adjusted life year.64 Similar cost-effectiveness for liraglutide was found in a Greek analysis, where liraglutide was associated with higher direct medical costs but an overall cost saving due to reductions in the treatment of diabetes-related complications.65 Taken together, these studies suggest that the use of novel diabetes agents may be cost-effective, but further study is needed to assess their role as primary treatment specifically for cardiovascular event reduction.
Conclusion
In the years since the paradigm-shift in diabetes drug approval, we have seen SGLT-2 inhibitor and GLP-1 receptor agonist trials demonstrate reduced cardiovascular events and mortality. It remains to be seen whether a similar paradigm-shift will follow in the way practitioners approach the management of patients with type 2 diabetes and comorbid cardiovascular disease. Moreover, questions remain regarding the cost-effectiveness of these medications. If the recent changes to society guidelines are any indication, we are likely to see cardiovascular disease risk become an important factor in diabetes drug selection going forward. As such, cardiologists should become familiar with these agents and the populations that stand to benefit from their use.