Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), causes approximately 60,000–100,000 deaths annually in the US.1
Major risk factors include recent hospitalization for surgery or acute medical illness (50–60%), followed by cancer (20%).1 PE is the third leading cause of cardiovascular death after MI and stroke.1 While VTE encompasses a broader spectrum of thromboembolic disorders, this review focuses specifically on DVT and PE, which are the most prevalent forms. In recent years, a transformative shift in acute care management occurred due to advancements in treatment and ongoing research efforts focused on improving supportive care. As our understanding of short- and long-term complications improved, a critical need to tailor patient management based on their risks emerged.
This review, focusing on VTE management in the critical care setting, explores the nuances in the diagnosis, risk stratification, and management of VTE based on developing trends in imaging assessment; the emergence of multidisciplinary PE response teams (PERTs); and the evolving treatment landscape, including percutaneous interventions and temporary mechanical circulatory support (MCS). Additionally, the review discusses the implications of right heart failure in PE and the evolving supportive care strategies.
Pulmonary Embolism
Diagnosis of Pulmonary Embolism
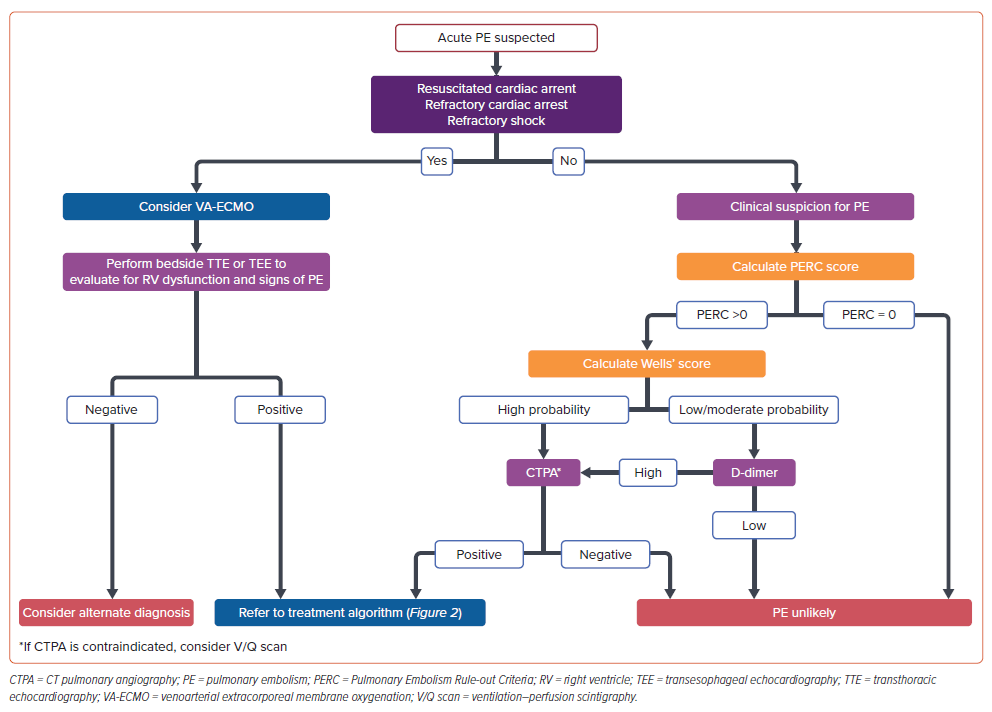
PE symptoms can range from mild to severe, and may include chest pain, dyspnea, syncope, or even cardiac arrest. Upon thorough clinical evaluation, the pulmonary embolism rule-out criteria score should be evaluated first. If the pulmonary embolism rule-out criteria score is 0, PE can be effectively ruled out without further testing. If the pulmonary embolism rule-out criteria score is not 0, the Wells’ score should be calculated. The Wells’ score stratifies patients into categories of PE probability (low, moderate, or high), thus guiding the subsequent decision-making process (Figure 1).2
D-dimer can be used to rule out PE in patients with low or moderate probability of PE owing to its high negative predictive value, but its low specificity limits its use as a confirmatory test, as the D-dimer can be elevated in other clinical scenarios, such as cancer, infection/inflammation, or during pregnancy.3 Contrast-enhanced CT pulmonary angiography should be performed for high suspicion or elevated D-dimer levels to visualize clot location and extent, assess right ventricular (RV) dysfunction, and evaluate parameters, such as right ventricle to left ventricle (RV/LV) diameter ratio (surrogate marker of RV strain), pulmonary artery (PA) enlargement, and contrast reflux into the vena cava.1,3–5 Although the RV/LV ratio is used as a surrogate marker for RV strain, its accuracy can be limited due to the complex 3D shape of the RV. While 3D imaging may offer improved accuracy, it is often impractical in acute settings where rapid evaluation is needed.6
Transthoracic echocardiogram can also be helpful in assessing RV function by evaluating for the presence of indices, such as increased RV/LV ratio, reduced contractility of the RV free wall compared with the RV apex (McConnell’s sign), 60/60 sign (presence of both a pulmonary acceleration time of <60 ms and a midsystolic ‘notch’ combined with a peak systolic gradient of <60 mmHg at the tricuspid valve), decreased tricuspid annular plane systolic excursion, and interventricular septum flattening. Although, it is important to keep in mind that echocardiographic signs of RV dysfunction can be seen in pre-existing cardiopulmonary diseases and are not specific to PE.3
Transesophageal echocardiography, if promptly available, is preferred to transthoracic echocardiogram due to its improved sensitivity for identifying PE, RV dysfunction, and detecting intracardiac thrombi.7 Moreover, transesophageal echocardiography is particularly valuable in critical situations, such as acute decompensation or cardiac arrest, as it can be integrated into active resuscitation efforts.8 While ventilation–perfusion scanning is less commonly used than CT pulmonary angiography in the acute setting due to its limited immediate availability, it remains a valuable diagnostic tool, particularly for patients who have contraindications to iodinated contrast media used in CT pulmonary angiography.2
After diagnosis, it is crucial to provide a preliminary prognosis through thorough examination, prompt assessment of mental status, hemodynamic profile, medical comorbidities (i.e. cancer, heart failure, and pulmonary disease), and the use of risk stratification tools.3,4,9 Following initial diagnosis and acute management, it is essential to discuss goals of care, including the consideration of palliative care when appropriate. Integrating palliative care discussions has been shown to enhance the quality of life for patients and their families, yet it remains underutilized in high-risk PE cases.10 Importantly, continuous clinical evaluation with close monitoring for changes in symptoms, heart rate, blood pressure, and oxygen requirement is necessary, as patients may shift from one risk category to another during their course.2
Risk Stratification
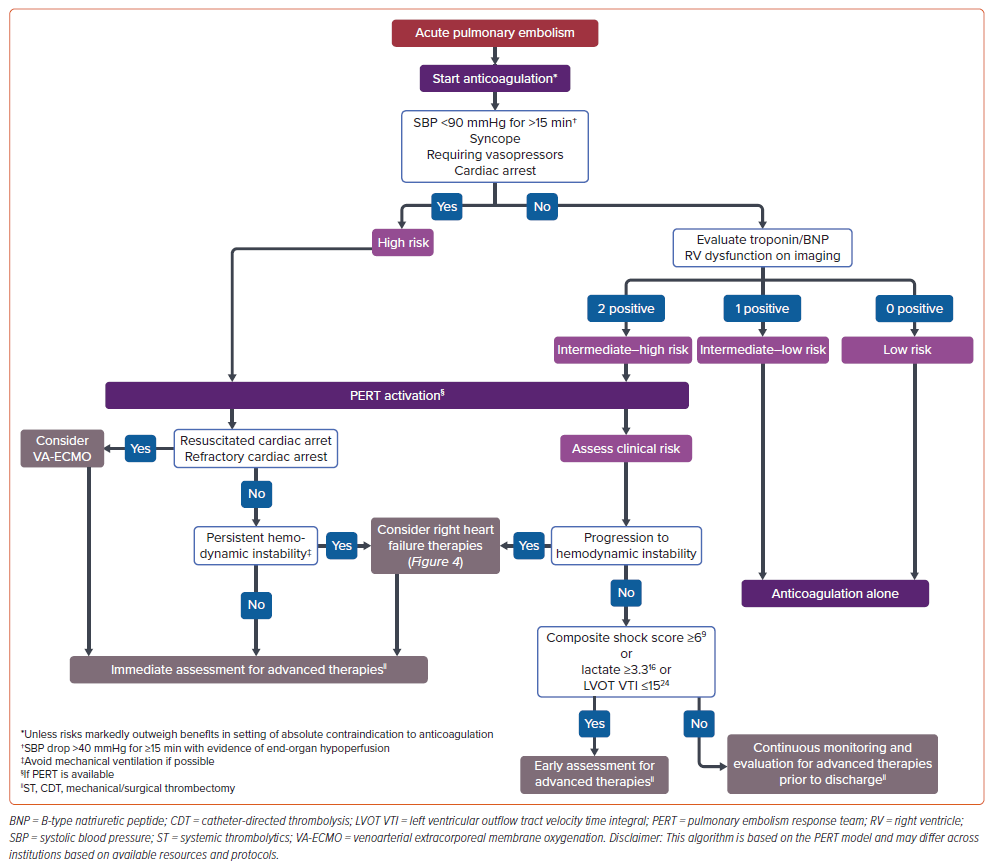
Acute PE can be stratified into three groups based on hemodynamic profile, imaging findings, and biomarker levels to guide mortality prediction and management. Guidelines categorize acute PE into low-, intermediate-, and high-risk PE, which have replaced the usage of the terms ‘submassive’ and ‘massive,’ which were previously used interchangeably with ‘intermediate risk’ and ‘high risk,’ respectively. Individuals who do not exhibit hemodynamic instability and signs of RV strain, and do not have elevated levels of cardiac biomarkers (troponin, brain natriuretic peptide/N-terminal pro-brain natriuretic peptide) are considered low risk. The high-risk category involves hemodynamically unstable patients, defined as those with syncope attributed to PE, post-cardiac arrest, a systolic blood pressure <90 mmHg, or a systolic blood pressure drop exceeding 40 mmHg for at least 15 minutes with evidence of end-organ hypoperfusion or use of vasopressors. Other etiologies of shock must be excluded.
Patients who are hemodynamically stable, but have either abnormal RV function or elevated biomarker levels, fall into the intermediate–risk category. The European Society of Cardiology subdivides intermediate risk further: abnormal RV or elevated biomarkers as intermediate–low, and the presence of both as intermediate–high risk.3,11 These stratifications aid triage and management decisions; however, with the recent surge in percutaneous interventions, there is an increasing need for more refined risk stratification tools to better identify the most suitable management strategies.
Mortality rates vary widely among patients who present with acute PE; high-risk PE is associated with an in-hospital mortality rate of 20.6%, which reaches 42.1% in those with hemodynamic collapse, while intermediate-risk PE is 3.7%, as reported in a contemporary analysis.12 Among patients with intermediate-risk PE, the prevalence of undiagnosed cardiogenic shock tends to be underestimated. Notably, the progression rate to hemodynamic instability is 5–6.5%, representing a cohort whose mortality closely aligns with the high-risk category.13 Moreover, 31.1% of patients with intermediate-risk PE, included in the FLASH registry, had a cardiac index ≤2.2, despite being normotensive. A composite shock score integrating various factors, such as cardiac biomarkers, central thrombus burden, concomitant DVT, and tachycardia, was developed to identify this cohort. Their findings highlighted that the prevalence of normotensive shock was 0% among patients with a composite shock score of 0, whereas a score of 6 emerged as a significant predictor of normotensive shock (OR 5.84). Factors, such as concomitant DVT, tachycardia, and BMI <30, independently increase the risk of normotensive shock. Notably, comorbidities, such as history of congestive heart failure, pulmonary hypertension, chronic obstructive pulmonary disease, or cancer, did not raise the risk.12
In accordance with a comprehensive registry dataset, high-risk PE was more likely to be associated with altered mental status, syncope, lower BMI, and a higher number of medical comorbidities than intermediate-risk PE. Moreover, high-risk PE complicated by hemodynamic collapse or cardiac arrest exhibited a higher likelihood of elevated serum troponin levels, fever, recent hospitalization, and requirement for vasopressors upon presentation. Furthermore, factors, such as vasopressor use, venoarterial extracorporeal membrane oxygenation (VA-ECMO) usage, clot-in-transit, hypoxia at presentation, and malignancy, are significantly associated with in-hospital mortality in high-risk PE patients. High-risk PE is also associated with a higher incidence of major bleeding and a longer hospital stay.14
Traditional scoring systems, including the Pulmonary Embolism Severity Index (PESI) and simplified PESI, which use age, vital signs, and medical history, have been found to have low specificities for early all-cause mortality (0.49 and 0.38, respectively).15 This diminishes their usefulness in the intermediate-risk category, which is associated with a higher risk of mortality. Notably, the risk of normotensive shock in intermediate-risk PE is not significantly different between those with a simplified PESI score 0 compared with a score ≥1.12 Comparison of the contemporary risk scores mentioned in guidelines and expert reports (PESI, simplified PESI, and Bova) showed only a moderate association with mortality risk, and a low association within these scoring systems.14
Elevated lactate levels have been linked to increased 30-day mortality in PE patients.16 Integrating lactate into the European Society of Cardiology 2019 algorithm enhances risk prediction and identifies patients at a higher risk for hemodynamic collapse. A lactate threshold of 3.3 mmol/l is a strong predictor of unfavorable outcomes in intermediate-high risk PE patients (OR 5.2; 95% CI [1.8–15.0]).17 Additionally, combining elevated lactate and Bova score resulted in a 24.1% incidence of hemodynamic collapse, outperforming Bova or TELOS risk scores alone, underscoring the potential benefit of incorporating lactate into risk scores.18
The impact of thrombus location has been a topic of debate. While peripheral clots were more likely to present with hypotension, central clot burden did not impact 90-day outcomes.19 However, a different study demonstrated a higher mortality rate associated with central clot burden.20 Additionally, saddle PE raised normotensive shock risk compared with unilateral PE in intermediate-high-risk PE (OR 6.81; 95% CI [1.27–36.50]).9
Focusing on echocardiography, tricuspid annulus plane systolic excursion ≤15 and clot-in-transit were shown to be predictors of 30-day mortality, whereas the RV/LV ratio was not an independent predictor.21,22 Importantly, LV outflow tract velocity time integral ≤15 and low RV outflow tract velocity time integral can indicate a higher risk of death and hemodynamic compromise.23,24 Although not extensively studied, echocardiographic markers, such as RV fractional area change, RV cardiac performance index, RV outflow track acceleration/deceleration times, and RV outflow track Doppler notching, may be beneficial in risk stratification.4
The mortality rate of PE patients remains high, despite therapeutic advancements and improved critical care management. The evolving treatment landscape necessitates updated risk scores, integration of additional parameters, and a standardized algorithm for precise stratification to enable the prompt initiation of tailored management strategies to prevent RV deterioration and excess mortality. Figure 2 presents an example of a stratification and management algorithm.
Establishment of Multidisciplinary Pulmonary Embolism Response Teams and Ongoing Challenges
The absence of established best practices, particularly for responding to intermediate- and high-risk PE patients, prompted multidisciplinary PERTs. Inspired by the acute stroke team and heart team models, they involve various specialties, including general cardiology, critical care, pulmonary, emergency medicine, interventional cardiology, interventional radiology, vascular surgery, vascular medicine, and cardiac surgery.4,25 The presence of PERTs has led to increased usage of percutaneous interventions, yet the lack of clinical trials to assess its clinical consequences remains an important gap.25 Furthermore, among hospitals with PERTs, there remains significant variability in the use of catheter directed thrombolysis (CDT), again reinforcing the need for more systematic approaches and randomized controlled trials to optimize patient assessment and care strategies.4 While current clinical practice guidelines advocate for forming multidisciplinary PERT teams in the management of high- and intermediate-risk PE patients, the lack of randomized trials evaluating PERT implementation makes the extent of its impact on survival, hospital length of stay, and costs uncertain.4,25
Management of Intermediate- and High-risk Acute Pulmonary Embolism: Overview
Anticoagulation remains an essential treatment in the current landscape of acute PE therapies. Systemic thrombolysis has a class 1 recommendation for the management of high-risk PE, but carries a notable risk of intracranial hemorrhage (ICH). In some institutions, surgical thrombectomy is feasible, but has its own risks. Percutaneous interventions have emerged over the past decade as part of the armamentarium in the management of acute PE, but lack extensive comparative data with long-term follow up.26 Current guidelines recommend mechanical thrombectomy, either percutaneously or via surgery, as a class 2b recommendation for high-risk PE.3 Likewise, percutaneous interventions for intermediate-risk PE patients have yet to exhibit a mortality advantage in randomized controlled trials. Nevertheless, the available data indicate that these interventions present a favorable safety profile, featuring a low risk of bleeding while promoting symptom improvement and reducing length of hospital stay.4 The following sections discuss the data leading to the current recommendations and emerging advanced therapies.
Management of Intermediate- and High-risk Acute Pulmonary Embolism: Pharmacotherapy
The PEITHO trial, the largest ST study in intermediate-risk PE patients, compared tenecteplase 30–50 mg with anticoagulation alone in 100 patients, showing a significant reduction in the composite endpoint of all-cause mortality and hemodynamic collapse within 7 days. Hemodynamic collapse was the main driver of the composite endpoint, as mortality rates did not significantly differ between groups. Moreover, tenecteplase resulted in more major bleeding and a 2% incidence of ICH.27 Subsequently, a meta-analysis of intermediate-risk and high-risk PE patients showed decreased mortality with ST, but also increased major bleeding and ICH. This reduction in mortality persisted in the intermediate-risk PE subgroup. Additionally, ST was associated with a 60% decrease in recurrent PE compared with anticoagulation alone.28
A Cochrane review of 21 trials and 2,401 high-risk or intermediate–high-risk PE patients found decreased mortality with ST. However, the results from the high-risk group were mainly driven by a small, randomized trial of only eight patients.29,30 Notably, both alteplase 50 mg administered over 2 hours and the currently recommended regimen of 100 mg IV over 2 hours yielded RV function improvement without a significant difference in the safety profile. This raises the question about whether the optimal dosing strategy for ST has been conclusively determined.31
Management of Intermediate- and High-risk Acute Pulmonary Embolism: Percutaneous Interventions
Catheter-directed Thrombolysis
CDT involves administering thrombolytics directly into the PA circulation through a catheter, providing the advantage of a markedly reduced thrombolytic dose. Through a targeted infusion into obstructive PA segments, CDT is thought to avoid the potential dispersion of thrombolytics to non-obstructive segments due to blood perfusion shunting seen with ST.4,32 Ultrasound-assisted CDT offers local thrombotic delivery and dissolution of fibrin strands via high-frequency, low-energy ultrasound.33 Although there is no direct comparison in pulmonary arteries, a study comparing the performance of CDT and ultrasound-assisted thrombolysis in iliac arteries showed no significant difference.34
To date, studies addressing the clinical efficacy and safety of CDT have focused on the RV/LV ratio and post-intervention PA pressure changes. Among those, the PERFECT registry, SEATTLE-2 single-arm study, and ULTIMA randomized controlled trial comparing CDT with anticoagulation alone demonstrated significant improvements in PA pressures compared with pre-intervention following CDT without reported ICH in a population mostly consisting of intermediate-risk patients, with some involvement of high-risk patients.35–37 The OPTALYSE-PE trial using ultrasound-assisted CDT revealed that lower doses of tissue plasminogen activator (range 8–24 mg) given over shorter infusion times (range 2–6 hours) resulted in improvements comparable to the larger 24-mg dose administered over longer infusion times (12–24 hours), as demonstrated by a marker of RV function.38
The results of a network meta-analysis comparing anticoagulation alone, CDT, and ST revealed that CDT was associated with lower overall mortality than anticoagulation alone or ST. This trend persisted, even when focused solely on intermediate-risk PE patients. While major bleeding rates were higher with CDT than with anticoagulation alone, they were similar to those with ST. Notably, ST was associated with a greater likelihood of ICH as compared with CDT (OR 1.50; 95% CI [1.13–1.99]). The incidence of ICH was higher numerically with CDT compared with anticoagulation alone (OR 1.51; 95% CI [0.75–3.04]), although the difference was not statistically significant.
These findings suggest that CDT may be an effective therapy for reducing mortality in patients with intermediate-high risk PE, while anticoagulation remains the safest strategy to avoid major bleeding among these three approaches.39
Percutaneous Mechanical Thrombectomy
Mechanical thrombectomy involves physically removing blood clots from the pulmonary blood vessels without employing thrombolytic medications. In the FLARE registry, mechanical thrombectomy, using the FlowTriever system (Inari Medical), reduced the RV/LV ratio by 25% in intermediate-risk PE patients. There was only one major bleeding event (1.0%), and the average intensive care unit stay was 1.5 days.40 The EXTRACT-PE trial, using a smaller bore aspiration catheter – the Indigo aspiration system (Penumbra) – also showed a significant RV/LV ratio reduction with minimal bleeding.41
In the FLASH registry, in the US cohort of 800 patients 76.7% had intermediate–high-risk and 7.9% had high-risk PE, and the results confirmed the efficacy and the enhanced safety profile of mechanical thrombectomy with on-table hemodynamic improvements, reduced intensive care unit need, and improved symptoms. Moreover, all-cause mortality was low, with a rate of 0.3% at the 48-hour follow-up.42 Among a subgroup of FLASH Registry patients diagnosed with normotensive shock, 30.5% showed a normalized cardiac index correlating with a reduction in RV size and improved quality of life at 30-day follow-up.9
Assessing the efficacy and safety of mechanical thrombectomy in high-risk PE patients, the FLAME study was a prospective, non-randomized, parallel-group observational study comparing mechanical thrombectomy with contemporary therapies (68.9% with STs and 23% with anticoagulation alone). The results significantly favored a mechanical approach to the management of high-risk PE, revealing in-hospital mortality rates of 1.9% for mechanical thrombectomy versus 29.5% for contemporary therapies, and highlighted lower in-hospital adverse outcomes with mechanical thrombectomy.43
Management of Intermediate- and High-risk Acute Pulmonary Embolism: Surgical Embolectomy
Surgical embolectomy is an alternative treatment strategy for increased-risk acute PE in patients with contraindications to thrombolysis. To date, the available data in this area remain limited.
A meta-analysis of high-risk PE patients supported on VA-ECMO compared the benefits and limitations of mechanical reperfusion (86% had surgical embolectomy) with other therapies, including ST, CDT, or anticoagulation alone. Compared with other therapies, mechanical reperfusion, particularly surgical embolectomy, showed improved mortality and lower bleeding rates irrespective of the presentation or timing of VA-ECMO cannulation. In the subgroup analysis, mechanical reperfusion group was superior to ST for mortality and bleeding.44 However, despite the advantages of surgical embolectomy, the data on this topic remain restricted, especially considering the increased use of percutaneous therapies in recent years.
Future Directions
For high-risk PE patients, the central debate centers around the primary approach: ST versus CDT and percutaneous or surgical mechanical thrombectomy with or without VA-ECMO support in combination with anticoagulation. Large registry data showed that high-risk PE patients compared with intermediate-risk PE patients were more likely to receive advanced therapies, such as ST, surgical embolectomy, and MCS. However, there was no difference in the use of catheter-based therapies between the two populations. Notably, those with hemodynamic collapse were more likely to receive ST and MCS with VA-ECMO, but less likely to receive catheter-based or surgical therapies, which was attributed to their increasing instability hindering alternative invasive procedures.11
Extrapolating from the evolution of the management of MI, treating high-risk PE patients aligns with ST-elevation MI care, advocating for an immediate invasive strategy. For intermediate–high-risk PE, similar to non-ST-elevation MI, an algorithm with a scoring system similar to the Global Registry of Acute Coronary Events score is needed to refine risk assessment and decide on the best timing for invasive strategies.45
Looking ahead, there are several trials evaluating treatment strategies in intermediate- and high-risk PE patients. It is crucial to recognize that, although the RV/LV ratio has traditionally been used as an indicator of RV dysfunction, recent research has shifted focus toward prioritizing outcomes, such as mortality and PE recurrence, as primary objectives.
The HI-PEITHO trial (NCT04790370) will compare ultrasound-assisted CDT plus anticoagulation with anticoagulation alone in intermediate–high-risk acute PE patients, with the primary outcome being a composite of PE-related death, cardiorespiratory decompensation or collapse, and non-fatal symptomatic PE recurrence.
The PEERLESS trial (NCT05111613) is assessing mechanical thrombectomy with the FlowTriever System (Inari Medical) versus CDT in hemodynamically stable patients with acute PE with RV dysfunction, using a hierarchical composite win ratio that includes all-cause mortality, intracranial hemorrhage, major bleeding, clinical deterioration, and intensive care unit admission. This is followed by PEERLESS II (NCT06055920), which compares mechanical thrombectomy with the FlowTriever System plus anticoagulation with anticoagulation alone for intermediate-risk acute PE patients, with a primary endpoint of a hierarchical composite win ratio, including all-cause mortality, clinical deterioration, hospital readmission, bailout therapy, and dyspnea score.
Additionally, the PE-TRACT trial (NCT05591118) is set to compare CDT plus anticoagulation with anticoagulation alone in intermediate risk acute PE patients, with primary outcome measures of peak oxygen consumption at 3 months, New York Heart Association classification at 12 months, and major adverse events.
Management of Pulmonary Embolism: Supportive Care for Right Heart Failure
Physiology of Right Ventricular Failure
The RV and LV have different mechanical and functional structures, which affects their abilities to respond to abrupt changes in preload and afterload. Compared with the LV, the more compliant RV is better able to manage preload changes. The RV is less able to respond to sudden increases in afterload, so there are more pronounced reductions in stroke volume with incremental increases in afterload.46
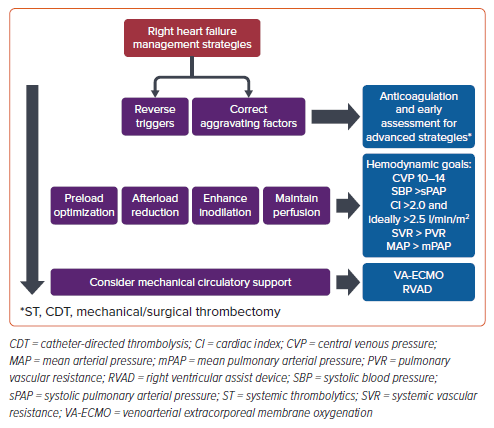
To mitigate the reduction in stroke volume, the RV dilates in an attempt to increase contractility using the Frank–Starling mechanism. This results in worsened tricuspid regurgitation, which causes the RV to dilate further. Because the two ventricles share the interventricular septal wall and the pericardial cavity, RV dilatation results in a leftward shift of the interventricular septum.47 The shift reduces LV diastolic filling, which further decreases cardiac output (CO), and worsens systemic and right coronary hypoperfusion. Since RV dilatation also results in increased wall tension and myocardial oxygen demand, the combination of diminished oxygen supply and augmented oxygen demand leads to RV ischemia.48 These detrimental physiological effects reinforce each other, resulting in catastrophic hemodynamic deterioration that is also termed the ‘RV death spiral’ (Figure 3).
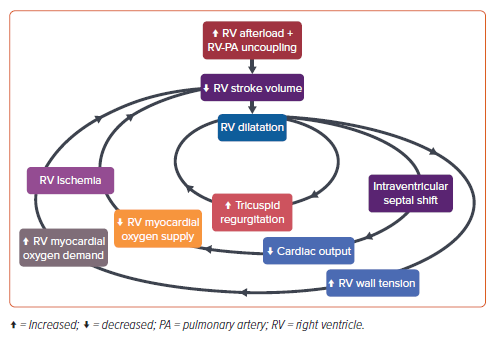
With the many physiological changes that occur with pulmonary emboli, measures of coupling can help to assess the adequacy of RV contractility against increased pulmonary arterial afterload. Coupling is identified as the energy transfer between ventricular contractility and arterial afterload, and can be mathematically interpreted as the ratio between end systolic elastance and effective arterial elastance.49 With acute pulmonary emboli, the arterial afterload (effective arterial elastance) acutely increases, which then reduces RV stroke volume (end systolic elastance). When the end systolic elastance/effective arterial elastance ratio decreases sufficiently (<1.0), the RV is considered to be uncoupled from the pulmonary arteries.50 In other words, the RV and PA are uncoupled when the RV cannot sufficiently increase contractility to match the increase in load from the PE in the pulmonary arteries. This is pragmatically captured by echocardiographic measures of coupling, such as the tricuspid annulus plane systolic excursion to RV systolic pressure ratio, which has been suggested to be predictive of adverse outcomes including all-cause mortality in acute pulmonary emboli.51
Management of Acute Right Ventricular Failure: Overview
The management of acute RV failure relies on optimization of RV preload, afterload, and contractility while maintaining adequate perfusion. Routine use of invasive hemodynamic monitoring with PA catheters is not recommended in heart failure.52 However, the direct measurement of PA pressures, biventricular filling pressures, and CO can offer important hemodynamic insights in the assessment and management of RV failure.53 While there are limited data to guide hemodynamic goals, expert opinion suggests aiming for hemodynamic parameters, including systemic vascular resistance (SVR) greater than pulmonary vascular resistance (PVR), mean arterial pressure greater than mean pulmonary arterial pressure, systolic blood pressure greater than systolic pulmonary arterial pressure, cardiac index sufficient to maintain perfusion (>2.0 and ideally >2.5 l/min/m2), and central venous pressure 10–14.54 As RV failure can progress rapidly, frequent reassessment and aggressive initiation of therapies is warranted (Figure 4).
A primary aim in managing RV failure is to identify and prevent further deterioration into the ‘RV death spiral’ (Figure 3). As previously discussed, the RV is particularly sensitive to changes in afterload. Metabolic states, such as hypoxemia, hypercarbia, and acidemia, can worsen PVR, so correcting such reversible etiologies is critical.55,56 In addition, careful consideration is needed before implementing any intervention that may adversely affect RV afterload and contractility. For example, intubation and positive pressure ventilation (PPV) can have catastrophic effects in right heart failure due the adverse cardiac impacts of sedation and PPV.57 It is critical to thoughtfully examine the physiology of all interventions, and we will discuss a few central principles in more detail below. Finally, while we discuss these topics separately, it is important to note that the urgency of the situation usually dictates quick sequential or concurrent initiation of therapies.
Management of Acute Right Ventricular Failure: Preload Optimization
Maintaining adequate preload is important in optimizing myocardial contractility due to the Frank–Starling relationship. However, excessive preload can reduce CO due to interventricular interdependence. In the case of acute PE, there is conflicting evidence supporting both fluid and diuretic administration.58,59 In light of this conflict, we advocate for leveraging central venous pressure measurements or surrogates for this measurement to guide preload management, and avoid both underfilled and overloaded volume states.3
Management of Acute Right Ventricular Failure: Maintenance of Perfusion
Adequate management of RV failure relies on the preservation of systemic and right coronary artery perfusion. Maintaining adequate systemic vascular resistance and pressures can limit the negative effects from ventricular interdependence by restricting the degree of septal shift.60 This optimizes CO, further supporting systemic and right coronary perfusion. Perfusion can be maintained using vasopressors with inotropic properties, such as norepinephrine and epinephrine.47,53 We advocate for using low doses of multiple vasoactive agents whenever possible, as opposed to escalating doses of a single sympathomimetic vasopressor. At higher doses, utility can become limited by increasing PVR and tachyarrhythmias.53 In addition, vasopressin at a low dose has been demonstrated to result in pulmonary vasodilatation in animal models.61,62 Vasopressin’s effect on PVR has been inconsistent in human data, but has been described to be safe in case reports of pulmonary hypertension.53
Management of Acute Right Ventricular Failure: Afterload Reduction
Although evidence is limited, for some patients, using partially selective pulmonary vasodilators may help reduce RV afterload, improve RV–PA coupling, and support CO. Since some pulmonary vasodilators may reduce systemic blood pressure and compromise RV perfusion,63 pulmonary vasodilators should be used after systemic perfusion and CO are optimized.53 In addition, intravenous pulmonary vasodilators may result in undesirable changes in ventilation–perfusion, resulting in mismatching.64 Inhaled formulations of nitric oxide and prostacyclins may mitigate this risk, as they will preferentially end up in ventilated lung units.53
Management of Acute Right Ventricular Failure: Inodilator Support
Inodilators can help support CO and end organ perfusion in a failing RV. Dobutamine and milrinone have both inotropic and vasodilatory effects, and have broadly been demonstrated to achieve similar clinical outcomes in advanced heart failure.65 Dobutamine at low-to-moderate doses (<5 mg/kg/min) increases contractility, reduces PVR and SVR, and improves RV–PA coupling.66 However, at higher doses, dobutamine can increase PVR.53 Milrinone, a phosphodiesterase-3 inhibitor, augments contractility while also causing pulmonary and systemic vasodilatation.47 In comparison with dobutamine, milrinone elicits a more substantial reduction in RV afterload, attributable to its potent pulmonary vasodilatory effects, which in turn reduces RV end-diastolic pressure, but its use can be limited by impaired renal clearance and its longer half-life.47 As a result, there is not clear evidence to use one inodilator over the other, and their use must be individualized in this population.
Management of Acute Right Ventricular Failure: Mechanical Circulatory Support
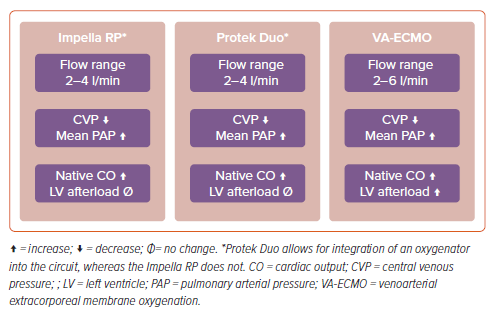
MCS is a therapeutic option for patients with high-risk acute pulmonary emboli in RV failure by directly or indirectly bypassing the RV. VA-ECMO indirectly bypasses the pulmonary circulation by draining blood from the right atrium and returning oxygenated blood to the systemic arterial circulation.67 Through this mechanism, VA-ECMO reduces RV preload, ultimately decompressing the failing RV and improving RV ischemia (Figure 5).68 While there are no randomized controlled data to support the routine use of VA-ECMO in high-risk PE, VA-ECMO has been promoted as potential life-saving therapy in various retrospective studies because of its ability to rapidly improve cardiogenic shock.69,70 However, this physiological advantage has not always been accompanied by a clear survival benefit. When VA-ECMO is used with anticoagulation alone, mortality rates are reported to be 69–78%.71,72 However, those who received surgical embolectomy or CDT in conjunction with VA-ECMO are reported to have lower mortality rates of 5–29%.71–73 A recent meta-analysis suggests that VA-ECMO does not offer a mortality benefit (OR 1.24; 95% CI [0.63–2.44]), although there was also considerable heterogeneity across the studies (I2=54%).74 Due to the limited evidence, the 2020 European Society of Cardiology guidelines conclude that VA-ECMO may be helpful in high-risk PE, and suggest that future prospective studies assess its benefit.3
RV assist devices offer an alternative method of mechanical support for those with acute RV failure. In contrast to VA-ECMO, they directly bypass the RV by removing blood from the right atrium and emptying into the pulmonary arteries, which reduces RV preload while increasing LV preload and CO (Figure 5). Through this mechanism, the RV is unloaded and end-organ perfusion is optimized. Data to support the use of temporary RV assist devices in acute pulmonary emboli have been limited to case reports and case series; there is not yet clear consensus on the clinical benefit.75,76
Heart–lung Interactions in Acute Right Ventricular Failure: Positive Pressure Ventilation Concerns
Positive pressure ventilation can affect RV preload, afterload, and myocardial perfusion, and should be meticulously managed. Similar to the LV, PPV reduces venous return and RV preload. In contrast, PPV can have differential effects on RV afterload. When lung volumes are too low and atelectatic, the collapsed lung units cause alveolar hypoxic vasoconstriction. As positive pressure opens collapsed areas of the lung, this lessens hypoxic vasoconstriction, which decreases PVR.57,77 If alveoli are overdistended, positive pressure can compress extra-alveolar vessels and ultimately increase PVR. In summary, PVR increases with either under- or overdistention of alveoli, either of which can be hemodynamically catastrophic in RV failure.57,78
However, while RV physiology can be adversely affected by PPV, reversing states of hypoxemia, hypercarbia, and acidemia with PPV may still improve PVR and RV function.
Due to the complexity of these effects, managing patients with RV failure on PPV can be especially challenging. To minimize the detrimental effects of PPV on RV failure, expert consensus recommends using ventilatory strategies that minimize alveolar under- or overdistention, avoid high plateau pressures (>30 cmH2O), hypoxia, and hypercarbia.57
Heart–lung Interactions in Acute Right Ventricular Failure: Intubation Concerns and Choice of Sedation
In patients with acute PE and RV failure, endotracheal intubation should be avoided if possible, not only because PPV can exacerbate RV failure and result in hemodynamic collapse, but also because many of the induction medications for intubation can adversely affect the failing RV. Several induction medications substantially reduce SVR, which is critical for RV perfusion. Without careful consideration of the medication choices, induction can result in the ‘RV death spiral’ (Figure 3). Some experts advocate for awake bronchoscopic intubations to strategically avoid systemic induction medications.79 If awake bronchoscopic intubations are not possible, we advocate for the careful consideration of induction agents and their hemodynamic impacts.
While there are no randomized trials to guide the use of any specific regimen, etomidate and ketamine are often used, given their more ‘neutral’ hemodynamic effects.80 Etomidate has minimal effects on CO and SVR.80,81 In earlier studies, ketamine was suggested to increase PVR and mean pulmonary arterial pressure, but has more recently been demonstrated to have minimal effect on PVR.82,83 While ketamine has been demonstrated to have direct negative inotropic effects, ketamine can also indirectly increase sympathomimetic activity and help bolster systemic perfusion. Therefore, in patients who are not chronically ill and catecholamine depleted, ketamine may offer an attractive alternative in the setting of a failing RV.84
Deep Vein Thrombosis
Acute Management
The guidelines endorse anticoagulation alone in the treatment of acute DVT, supported by moderate certainty of evidence.3 Although this is the current standard of care, the high incidence of post-thrombotic syndrome (PTS), which affects 20–50% of DVT patients, suggests that current treatment strategies may be inadequate. This persistent burden raises important questions about whether relying solely on anticoagulation is sufficient, and calls for a re-evaluation of management strategies for DVT. PTS can range from mild symptoms to chronic debilitating leg pain and ulcers, potentially necessitating intensive medical care and significantly diminishing quality of life. Older age, obesity, and proximal thrombus location, particularly in the common femoral or iliac veins, are significant risk factors for PTS.85
A Cochrane review, which encompassed the COVANT, ATTRACT, and CAVA trials, revealed that CDT demonstrated greater efficacy in achieving complete clot dissolution of DVT in the lower limb compared with anticoagulation alone. However, while the usage of CDT was associated with a slight decrease in the incidence of PTS, there was an increase in bleeding complications.86 Recently, the CLOUT Registry’s 6-month data showed promising efficacy and safety of mechanical thrombectomy in the management of lower extremity DVT, with significantly improved PTS and enhanced quality of life, including similar improvements in both iliofemoral and femoral popliteal segments.87 A propensity score-matched analysis comparing mechanical thrombectomy with CDT in the management of lower extremity DVT demonstrated greater periprocedural thrombus reduction and improved iliofemoral vein disease symptoms with mechanical thrombectomy. At 12 months, 83.1% of patients were free of PTS with mechanical thrombectomy, compared with 63.6% with CDT (p=0.007).88
Surgical venous thrombectomy has a class 2B recommendation for proximal DVT.3 A meta-analysis (included eight trials from the 1970s through the 1990s) showed a 33% risk reduction in PTS with surgical thrombectomy compared with systemic anticoagulation.89 However, no recent trials compare surgical thrombectomy with CDT or mechanical thrombectomy.
Despite promising results from some studies, there remains a current lack of robust evidence supporting the routine use of percutaneous therapies for DVT management. Further research is needed to clarify their role in improving long-term outcomes and reducing complications, such as post-thrombotic syndrome.
Inferior Vena Cava Filters
The utility of inferior vena cava filters are debated due to studies revealing their overuse.90 They may be valuable in acute VTE with contraindications to anticoagulation, progressive VTE despite adequate anticoagulation, or as an adjunct to anticoagulation for severe VTE burden. Nevertheless, routine use is not advised because of associated risks, such as fracture, embolization, and increased DVT likelihood. Moreover, studies to date found no significant decrease in PE risk with inferior vena cava filters as an adjunct to anticoagulation.91 When safe, retrieval of these devices upon resolution of the initial reason for placement and along with initiation of anticoagulation is recommended, but often overlooked.10,92
Conclusion
Managing intermediate- and high-risk acute PE in critical care units necessitates the prompt recognition of predictors associated with high morbidity and mortality. Assessment of the initial vital signs, risk markers, and appropriate imaging modalities can be helpful in this regard. Advanced therapies, such as percutaneous options, surgery, or systemic thrombolytics, should be evaluated on a case-by-case basis in a timely manner, with the involvement of PERTs. The critical care physician should be on the alert for any sudden increase in RV afterload, as it can be detrimental. Continuous aggressive adjunctive supportive care is paramount to prevent progression toward a downward RV spiral.
Managing RV failure in acute PE involves preload optimization, afterload reduction, and contractility support to maintain perfusion. Intubation should be avoided when possible, as certain induction medications and PPV can have untoward effects on the RV. MCS options, such as VA-ECMO and RV assist devices, should be considered, although limited data support their use in acute PE scenarios. Additionally, it is crucial to evaluate patients with PE for DVT and administer appropriate treatment, as prompt identification can have a significant impact on the patient’s overall treatment plan and prognosis. Future advancements in the management of acute VTE are anticipated through ongoing trials that evaluate approaches to optimize outcomes.