Hemodynamic assessment remains the cornerstone of accurate diagnosis of shock and the assessment of the response to therapy in critically ill patients. Contemporary cardiac intensive care units (CICU) manage patients with multiple co-morbidities along with an ailing heart.1–3 An increasing number of patients with septic shock and undifferentiated shock are treated in the CICU in conjunction with patients with cardiogenic shock (CS).4,5 For this reason, rapid and accurate hemodynamic assessment is essential for the differentiation of shock and subsequent guidance for treatment including escalation to pharmacological therapies or temporary mechanical circulatory support (MCS).6
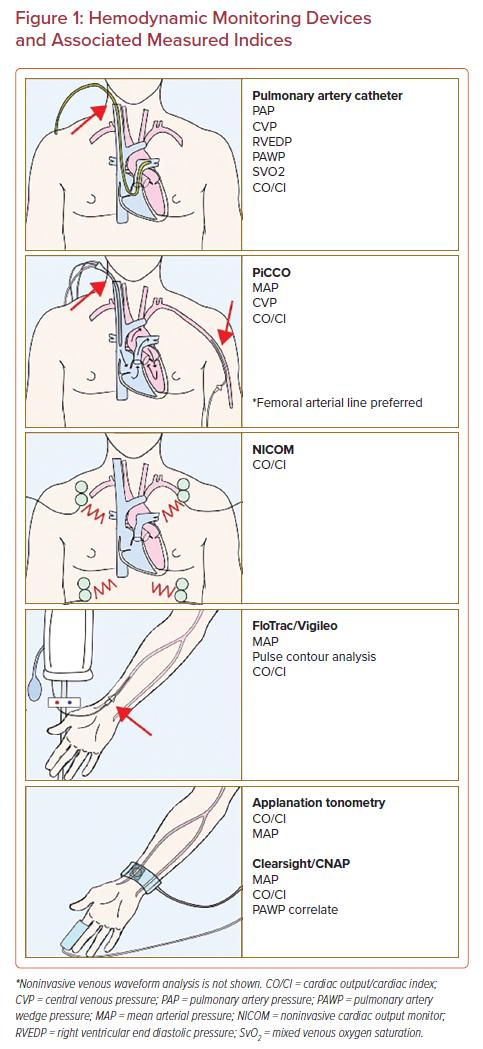
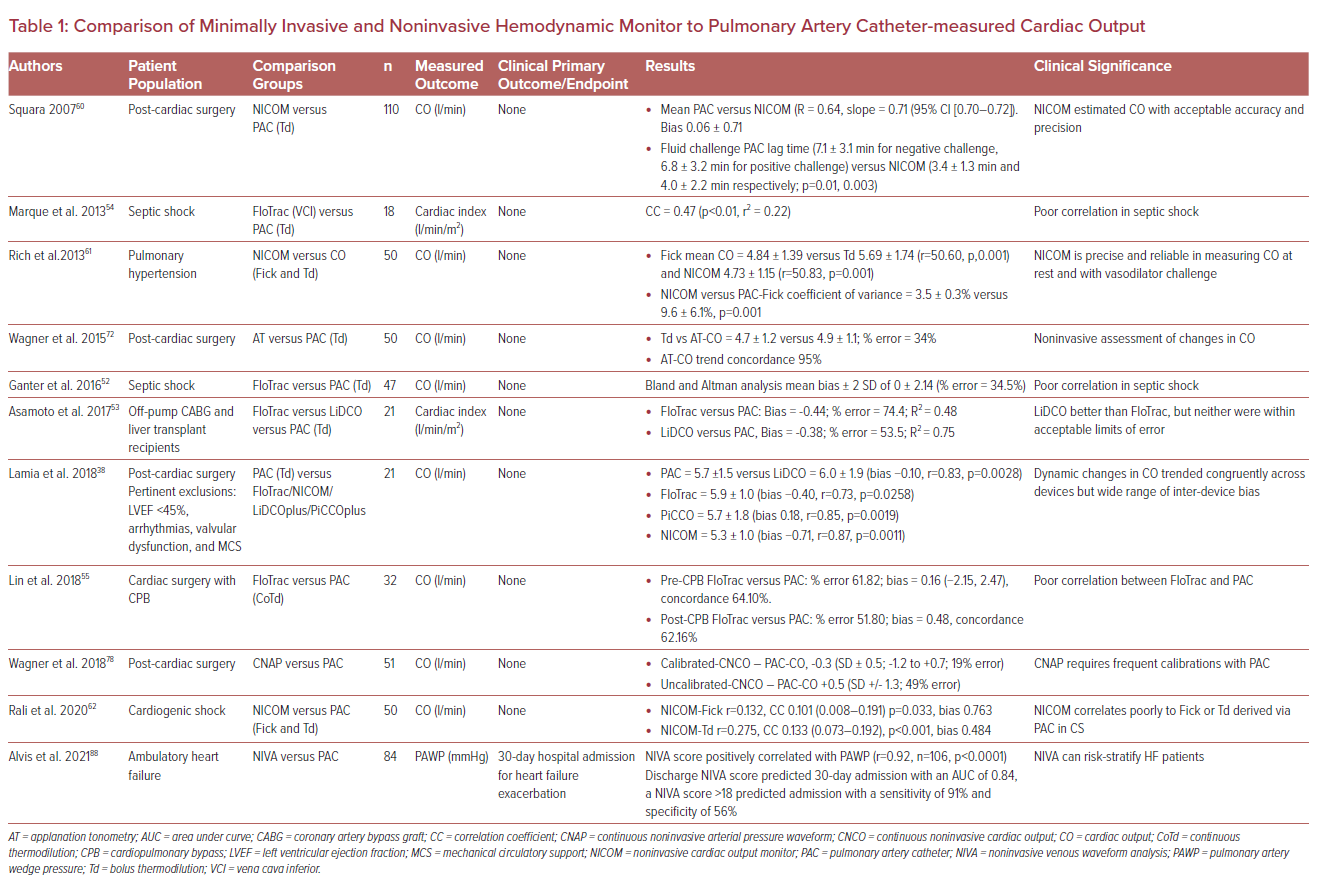
A myriad of invasive, minimally invasive, and noninvasive hemodynamic assessment modalities exists that supplement physical examination (Figure 1 and Table 1). These techniques rely on various physiological principles and assumptions to measure hemodynamic parameters. The aims of this review are to summarize the available literature on the mechanisms and clinical validity of various hemodynamic monitoring modalities as well as providing a contemporary update on pulmonary artery catheter usage. We have characterized each modality based on its level of invasiveness.
Physical Examination in Shock
The bedside physical examination is the oldest method of patient evaluation and can detect the presence of shock. Skin mottling, cool extremities, and delayed capillary refill have been correlated with mortality in patients with septic shock.7,8 Similarly, physical examination findings help grade severity of CS. Created in 1967, the Killip Classification grades heart failure (HF) severity post-MI based on progression from the presence of an S3 gallop or isolated rales, to pulmonary edema, to overt CS.9 The 2019 Society for Cardiovascular Angiography and Interventions (SCAI) Clinical Expert Consensus Statement on Classification of Cardiogenic Shock was developed based on physical examination in conjunction with biochemical and hemodynamic profiles. The SCAI physical exam can detect the worsening of CS on a continuum from isolated tachycardia and elevated jugular venous pressure; to cool extremities, pulmonary rales, oliguria, altered mentation, and narrow pulse pressure; to peri-arrest and arrest.6,8
While physical examination certainly has a part to play in the initial diagnosis of shock and the degree of shock severity, it may not be reliable in differentiating shock etiology. As early as 1984, Eisenberg et al. noted the pitfalls of physical diagnosis when they reported that physicians could only accurately estimate a pulmonary artery wedge pressure (PAWP) by physical diagnosis 30% of the time compared with pulmonary artery catheter (PAC) monitoring.10 Similarly, a 1994 study by Mimoz et al. demonstrated 56% accuracy in predicting patient hemodynamic profiles by physical exam alone.11 More recent observational studies in adults and children have had similar diagnostic inaccuracy.12,13 Evidence to date suggests that while it is an important screening index for the presence and severity of shock, physical examination alone is not adequate to determine the cause of shock nor risk stratification.14
An Update on Pulmonary Artery Catheters
PAC has been used for the direct measurement of hemodynamic profiles for several decades. It provides direct measurement of intracardiac pressures as well as estimates of cardiac output (CO) and cardiac index. PAC allows for the estimation of CO by two techniques – indirect Fick and bolus thermodilution (Td). Each has its pitfalls but Td is preferred over indirect Fick even in low output and severe tricuspid regurgitation.15–18 While measuring Td, it is critical that injections should be made in triplicate and all values within 10% of each other to account for beat-to-beat and manual injection variabilities. Furthermore, injection should occur at the same point of the respiratory cycle for consistent measurements.19
The original 1976 Forrester hemodynamic classification categorizes shock and CS treatment based on PAWP (‘wet’ versus ‘dry’) and cardiac index (‘warm’ versus ‘cold’) alone, thereby installing the PAC as a cornerstone of early CS management.20 Once enshrined as a permanent fixture in the management of intensive care patients, the PAC then became a focus of intense debate in the early 2000s after studies noted an increased rate of complication without a clear reduction in mortality.21–23
The 2005 ESCAPE trial then sought to determine the safety and efficacy of routine consecutive day use of PACs in patients hospitalized for chronic decompensated HF, and ultimately found no mortality benefit.24 It is worth noting that although the ESCAPE trial was a negative study, it was not focused on patients with general decompensated HF rather than the management of a CS population. The average systolic blood pressure in the study cohort was 106 mmHg and a very small percentage (<5%) would have met the clinical definition of CS. Although it did not meet its primary endpoint, ESCAPE’s secondary functional endpoints consistently favored PAC-directed therapy, especially in exercise capability and quality of life.24 However, subsequent meta-analyses of the use of PAC concluded a lack of mortality benefit with PAC placement and even a trend towards harm.25,26 In addition to unclear mortality benefit, PACs have been criticized for invasiveness and increased use of resources when there are potential alternatives, such as less invasive hemodynamic diagnostic devices.27
PAC is a diagnostic tool, not a treatment modality. As any other diagnostic tool, it cannot improve mortality by its mere placement. However, appropriate interpretation of real-time PAC hemodynamic profiles can easily capture hemodynamic changes while delineating the relative contributions and severity of right ventricular (RV) versus left ventricular (LV) failure in CS. Nuanced interpretation can then guide appropriate treatment strategy including initiation of inotropes as well as escalation to MCS therapy. Garan et al.’s analysis from the Cardiogenic Shock Working Group cohort found that complete PAC hemodynamic profiling in CS was associated with lower in-hospital mortality across all SCAI classifications even when adjusted for CS etiology, presence of MCS, and local PAC usage trends (adjusted OR: 1.57; 95% CI [1.06–2.33]). This study also found that an incomplete PAC hemodynamic profile portended similar in-hospital mortality risk to having no PAC profiling at all, which they theorized may have been due to an underestimation of RV contribution to CS.28 Accordingly, the most recent European Society of Cardiology guidelines classify clinical presentations of acute HF not only by Forrester-based CO and PAWP, but also by the presence of elevated RV end diastolic pressure.29
The ESCAPE trial showed potential benefits of PAC in high volume centers, perhaps a reflection of its usefulness among those who are more experienced with hemodynamic evaluation.11 By allowing medical providers to make better informed decisions, PAC could ultimately improve mortality in patients with CS. In an observational study by Ranka et al. analyzing the National Readmissions Database for patients admitted with acute CS (n=236,156), PAC-guided therapy was associated with a significant (31%) reduction in mortality during index hospitalization, a 17% reduction in 30-day HF readmissions rate and sixfold increase in usage of an LV assist device and orthotopic heart transplants during readmission.30 Hernandez et al. also found that despite the recent decline in the use of PAC in patients with CS, treatment guided by PAC assessment resulted in lower mortality during index hospitalization (n=915,416; 35.1% versus 39.2%, OR 0.91, 95% CI [0.88–0.95]; p<0.001).31 These, among other recent studies, have certainly revived discussion and debate about the need for appropriately interpreted PAC profiles as a powerful tool in CS management.32,33 While these studies have advocated for the role of PAC hemodynamic assessment in CS, randomized controlled trials will help solidify it.
Minimally Invasive Hemodynamic Monitoring
Pulse Index Continuous Cardiac Output Monitoring
Transpulmonary thermodilution or lithium dilution devices, such as the pulse contour CO (PiCCO) monitoring system (Pulsion Medical Systems/Getinge) and the LiDCO system (LiD-COplus, LiDCO), estimate CO by transthoracic thermodilution and lithium indicator dilution, respectively.34,35 They are less invasive than PAC in that they do not transverse the heart, but they still require central access. PiCCO is performed by injecting a cold fluid bolus via a central venous catheter and measuring the resultant thermodilution via a thermistor-tipped femoral artery catheter.27 The thermodilution curve (blood temperature versus time) translates to estimated CO by the Stewart–Hamilton equation. CO measured by PiCCO has been shown to be within acceptable agreement (r=0.97, p<0.0001) with PAC-based intermittent bolus thermodilution estimation of CO in critically ill patients.36 Once calibrated with thermodilution, PiCCO algorithmically incorporates pulse contour analysis for continuous CO and stroke volume variation measurement, quantitative estimation of extravascular lung water (EVLW), and other calculated hemodynamic parameters. However, frequent recalibration is required.37
In a study of 20 patients admitted to the intesive care unit (ICU) after cardiac surgery with arterial line and PAC monitoring, cross-comparison of PAC derived CO was performed with PiCCO and LiDCO estimations; mean CO measurements were similar, though accuracy suffered during dynamic changes in CO.34 A newer cross-comparison between PAC, PiCCO, and LiDCO devices demonstrated tight inter-device measurement of dynamic CO trends in post-cardiac surgery patients without significant cardiac dysfunction, arrhythmia, or valvular abnormalities (PAC-PiCCO r=0.85, p=0.0019; PAC-LiDCO r=0.83, p=0.0028), suggesting that prior inaccuracies may have been algorithmically corrected.38 Among patients with CS, studies comparing PAC to PiCCO found adequate concordance with the cardiac index, including in patients with valvular abnormalities or arrhythmias.39,40 PiCCO has also been shown to demonstrate concordance with transthoracic echocardiography in estimating cardiac output.27,35
Location of central venous catheter as well as presence of MCS devices can affect the accuracy of PiCCO measurements. Herner et al. described significantly lower estimations in cardiac functional index when catheters were placed in a femoral location instead of gold standard jugular or subclavian venous access, though later iterations of PiCCO monitoring algorithms have some provisions to correct for venous catheter location.41 Thermodilutional-derived global ejection fraction has shown more accuracy to date than thermodilutional-derived cardiac functional index regardless of venous catheter location.41,42 PiCCO accuracy can also be affected by MCS, such as intra-aortic balloon pump (IABP) counter-pulsation.39 The device detects every augmentation during IABP support as a new systole, resulting in inaccurate estimation of heart rate. Literature regarding PiCCO monitoring with other forms of MCS such as ventricular assist devices or veno-arterial extracorporeal membrane oxygenation is sparse.
More recently, PiCCO monitoring has also been used with adjunct carotid tonometry in the measurement of effective arterial elastance (Ea), which is defined as the ratio between central end-systolic pressure and stroke volume. Ea has been proposed as an alternative to systemic vascular resistance when measuring LV afterload and measuring the ventricular-arterial decoupling that occurs in shock states.6,43
The PiCCO system can provide a qualitative estimate of EVLW and has been proposed as a tool in management and prognostication of acute lung injury, acute respiratory distress syndrome (ARDS), and cardiogenic pulmonary edema.27,44 As the cold saline bolus is injected, the downslope of the thermodilution curve is used to estimate total pulmonary and thermal volumes, and EVLW is then estimated as the difference of intrathoracic blood volume and intrathoracic thermal volume.27,45 Targeting EVLW in sepsis and ARDS management has not revealed benefit. A multicenter randomized controlled trial of 350 patients demonstrated no mortality benefit of EVLW versus central venous pressure (CVP)-guided fluid balance in septic shock or ARDS.46 Indeed, little correlation is reported between EVLW estimates and shock subtype or ICU mortality.47,48 Given the evolution of fluid balance assessment in recent years, larger-scale prospective studies will be critical in determining the utility of EVLW estimations with PiCCO monitoring in critically ill patients.
Similar to PiCCO, the LiDCO system provides CO measurements by lithium indicator dilution generating a curve of concentration over time. A lithium chloride indicator is injected in either a central or peripheral venous line, then arterial concentrations of the lithium are measured by serial blood draws through an arterial line sensor.49 With three sequential dilution measurements, the coefficient of error in measurement of CO is as low as 5% in hemodynamically stable, ventilated intensive care patients.50 Initial inaccuracies reported during dynamic CO shifts seem to have improved in later algorithms, though notably patients with severe cardiac dysfunction (LV ejection fraction [LVEF] <45%), MCS, valvular dysfunction, and arrhythmias were excluded.34,38 It remains unclear to what extent these hemodynamic assessments affect clinical outcomes. Furthermore, an important caveat is that these systems only assess CO and do not provide the complete hemodynamic picture (including pulmonary artery pressure, PAWP, etc.) which is more valuable than any one parameter alone.28
Uncalibrated Pulse Contour Analysis
The FloTrac/Vigileo system (Edwards Lifesciences) uses pulse contour analysis derived from the arterial line to estimate stroke volume. When combined the patient’s demographic data via the Vigileo monitor, it can also provide estimations of CO, cardiac index, and stroke volume variation with suboptimal accuracy.35 This technique does not require calibration with PAC-measured CO but also does not provide estimates of intra-cardiac pressures such as CVP, PAP, or pulmonary capillary wedge pressure.35,51
FloTrac has been criticized for poor correlation with PAC measured CO, with a widely variable percentage error (up to 68%) across all generations of monitors and across several studies and settings (ICU, postoperative, septic shock patients).51–56 While its utility in accurate estimation of cardiac index is limited, it may be useful in trending change in cardiac index as a mark of volume responsiveness.57–59
Noninvasive Hemodynamic Monitoring
Noninvasive Cardiac Output Monitoring
Noninvasive Cardiac Output Monitor (NICOM, Cheetah Medical) measures intrathoracic bioimpedance by alternating AC currents through thoracic pulsatile blood flow. It then indirectly calculates the stroke volume as the derivative of the change in the NICOM signal amplitude between systole and diastole. This measurement is dependent upon the diffusion of oscillating electric currents through the thoracic cavity.
Studies evaluating the validity of the use of NICOM when compared with PAC have yielded mixed results. Squara et al. assessed CO in post-cardiac surgery patients by both NICOM and thermodilution by PAC and demonstrated that NICOM was a reliable method of measuring CO in this cohort.60 Rich et al. then demonstrated that NICOM was comparable to PAC in precision when assessing hemodynamics in patients with pulmonary artery hypertension.61 However, NICOM has not been reliable in assessing hemodynamics in patients with CS and acute decompensated HF. In a cross-sectional prospective clinical trial, Rali et al. found that NICOM correlated poorly with indirect Fick and thermodilution measurements of CO in patients with CS.62 It is plausible that these errors in measurement may be a result of interstitial and pulmonary edema and increased preload states in patients with chronic HF and low flow state in CS. The correlation did not improve with normalization of the cardiac index >2.2 l/min/m2 or with the achievement of euvolemic status (CVP <5 mmHg or pulmonary artery systolic pressure <25 mmHg).62
Arterial Applanation Tonometry
Arterial applanation tonometry noninvasively estimates the aortic pressure waveform as a correlate of cardiac hemodynamics. It is performed by securing a pressure sensor (tonometer) over the wrist to partially flatten the radial artery and capture the arterial pulse. The resultant pulse waveform then undergoes a Fourier transformation algorithm to estimate a central aortic pressure waveform.63 Since the arterial pressure waveform contour is primarily determined by the force and duration of ventricular ejection, aortic impedance, and peripheral vasculature resistance it can be calibrated to estimate hemodynamics including CO. The T-lineÒ system (Tensys® Medical) is a well-known applanation tonometer that estimates hemodynamics by formulaically auto-calibrating a pulse contour analysis of radial tonometry based on demographic and biometric patient data.35,64,65
Arterial tonometry has demonstrated accuracy in measuring beat-to-beat blood pressure variation and mean arterial pressure (MAP) in anesthetized surgical patients and critically ill non-cardiac patients.66–69 However, in critically ill patients with severe HF, arrhythmias, or valvular disorders, MAP estimations with arterial tonometry are less accurate than traditional arterial line monitoring, with a near 40% error reported.70 Small proof-of-concept studies comparing CO estimations of arterial tonometry to PAC in critically ill patients found that appropriately positioned and calibrated arterial tonometers were able to estimate CO with a 23–34% margin of error.71,72 However, a follow-up study comparing arterial tonometry to PAC measurements in patients undergoing major abdominal surgery had an error rate of 43%.73 Applanation tonometry is strongly affected by vasoactive medications, obesity, and arrhythmias, and loses precision in large hemodynamic shifts or changes in vascular tone.69,70
Arterial tonometry is gaining traction for hemodynamic estimations in an ambulatory setting, with promising application in screening for hypertension, obstructive sleep apnea, coronary artery disease, and LV hypertrophy, among other pathologies.63 It also has potential as an alternative to Doppler ultrasound to measure blood pressure in patients with an LV assist device and may be useful as a continuous wearable device.74,75 However, further improvements are needed for it to have consistently accurate arterial blood pressure and CO estimates in critically ill patients.
Volume Clamp Method-derived Pulse Contour Analysis
ClearSight (Edwards Lifesciences) and Continuous Noninvasive Arterial Pressure Waveform (CNAP, CNSystems) noninvasive hemodynamic measuring systems estimate CO via photoplethysmography of the finger pressure arterial waveform. In these volume clamp method devices, an occlusive band around the finger regulates the external pressure needed to keep a continuous arterial blood volume in the finger throughout systole and diastole.27 The resultant pulse contour analysis is then used to estimate CO and stroke volume variation.
In the surgical setting and in hemodynamically stable ICU patients, ClearSight and CNAP have demonstrated an approximate 25% margin of error when calibrated with thermodilution, and 25–45% error when auto-calibrated.73,76–78 However, ClearSight and CNAP are not usually thermodilutionally calibrated in clinical practice, and larger ICU studies found much higher margins of error and standard deviations of measurement in auto-calibrated ClearSight measurements of undifferentiated shock patients.79,80 ClearSight and CNAP are particularly affected by hemodynamic shifts that require recalibration, vasopressor use, arrhythmias, and peripheral arterial disorders, which may limit their broad application in accurate hemodynamic assessment of critically ill patients.77,81
More recent data demonstrate that ClearSight and CNAP may be useful to track fluid responsiveness. Boisson et al. found that thermodilutionally calibrated ClearSight versus PiCCO in the operating room accurately trended increase in CO after 250 ml fluid boluses.77 In hemodynamically unstable patients, auto-calibrated ClearSight was able to trend increase in MAP and cardiac index over time with fluid resuscitation of patients in the emergency room or rapid response.82,83 Similarly finger photoplethysmography has been used to measure pulse amplitude ratio, defined as the ratio of pulse pressure at the end of a Valsalva maneuver to before the onset of Valsalva, which can estimate PAWP in HF patients as well as help identify hospitalized HF patients at increased risk of 30-day HF events.84,85
Noninvasive Venous Waveform Analysis
The high capacitance, low compliance venous system has not been widely studied in noninvasive hemodynamic monitors to date due to limitations in collecting and measuring low-frequency venous signals. Noninvasive venous waveform analysis (NIVA) has recently been used to measure venous distension, and thereby estimate volume status and PAWP.
NIVA technology uses piezoelectric sensing over the superficial wrist veins to detect and amplify venous signaling, then applies a Fourier transformation and algorithm to the signal to estimate PAWP.86 An initial study comparing NIVA estimates of PAWP to right heart catheterization measurements demonstrated a sensitivity of 80% and specificity of 53% in detecting a PAWP of >18 mmHg.87 With further refinement, NIVA technology may be used as an adjunct or alternative to implantable PAP monitoring systems such as CardioMEMS, which have in turn shown to be useful in ameliorating HF exacerbations and hospitalizations.87–89 NIVA has also been proposed as a method to direct volume removal during hemodialysis.86 However, inpatient application of NIVA is yet to be evaluated.
Transthoracic Echocardiography
Critical care echocardiography (CCE) has gained significant popularity with increased availability of mobile echocardiography machines and training opportunities.90 The noninvasive nature of CCE is especially appealing and echocardiography has long been validated as a reliable measure of hemodynamics.91,92
While the full scope of CCE application exceeds the limits of this review, it is worth noting that CCE can estimate all advanced hemodynamics with relative accuracy and tracking aortic velocity time index (VTI) is a reliable means to track change in CO over time or in response to fluid administration.93,94 CCE also adds vital information about cardiac structural details such as regional wall motion abnormality; valvular pathology; and diastolic dysfunction.93 Echocardiographic findings help with appropriate interpretation of hemodynamic data, such as tricuspid regurgitation affecting interpretation of CVP. Jentzer et al. recently discovered that LVEF at admission measured by formal transthoracic echocardiography in acute HF correlated to SCAI shock stages (p<0.001 across all stages) and independently predicted mortality based on LVEF and E/e’ ratio.95 This study prompted renewed discussion about the need for invasive hemodynamic monitoring if echocardiographic-derived hemodynamic measurements not only provide the above-mentioned benefits, but also demonstrate strong correlation to shock stage.96 However, echocardiography only provides a single snapshot into the hemodynamic profile of a patient which, while extremely valuable, may change rapidly in the intensive care setting. In complex cardiac patients, CCE and continuous invasive hemodynamic monitoring such as PAC may then serve the most value when used to both detect and diagnose shock evolution.
Appropriate training and competency among non-ultrasonographers remain the most significant limitation in widespread CCE usage. While there are CCE training programs provided by several professional organizations, there is no current formal consensus on number of training hours or exams needed to ensure competency.97 In response to this, the American Society of Echocardiography has recently developed a Critical Care Echocardiography board certification to attempt standardization of CCE skills.98
Conclusion
Several minimally invasive and noninvasive modalities exist to assess hemodynamic parameters. Most of these modalities still require optimization and validation for widespread usage. In the interim, comprehensive invasive hemodynamic profiling of patients in shock with echocardiography, and in select cases, PAC – which overall does not appear to improve clinical outcomes – remains pivotal in ensuring timely diagnosis and optimal treatment, especially in the increasingly complex patient population of the modern day CICU.