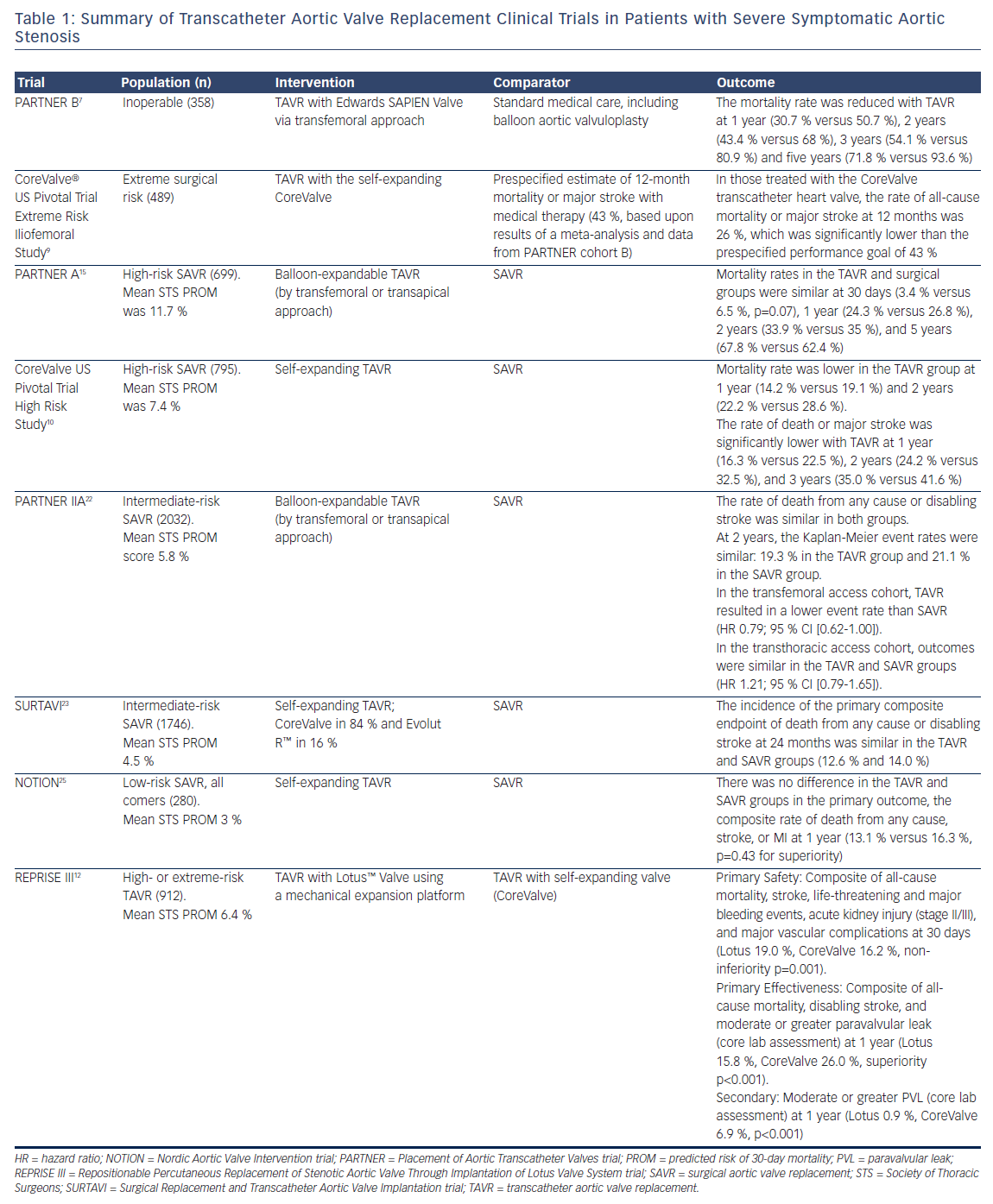
Aortic stenosis (AS) has a long latent period and then rapid progression with high mortality once symptoms appear.1 The absence of an effective medical therapy for symptomatic severe AS led to a class I indication for aortic valve replacement (AVR) in both the US and European guidelines.2,3 Unfortunately, a study by Bach et al. in 2009 found that up to half of patients with symptomatic severe AS were not offered surgical aortic valve replacement (SAVR) as they were not considered reasonable surgical candidates based on age, comorbidities, frailty, or other anatomic risks.4 Transcatheter aortic valve replacement (TAVR) was developed as a less invasive alternative to SAVR to allow treatment in this higher-risk patient population. Since the first successful TAVR in 20025 more than 250,000 procedures have been performed worldwide with a steadily decreasing patient-risk profile (Table 1).
Based on data from the original Placement of Aortic Transcatheter Valves (PARTNER) trials and CoreValve® US Pivotal trials, TAVR is now approved and accepted in the treatment for severe symptomatic AS in extreme-, high-, and intermediate-risk patient populations. Thus far, the randomized controlled trial data for TAVR have been non-inferior or even superior to both medical therapy and SAVR. Given the evolution of the data, the next step is to study low-risk patient groups. Anecdotal and non-randomized data have been conflicting when comparing TAVR with SAVR in low-risk patients. Two low-risk randomized trials have started in the US, and ultimately, these trials will determine the feasibility of TAVR as an acceptable alternative to SAVR in low-risk patients with severe AS.
Extreme-risk Patients
The onset of symptoms in severe AS heralds the usually rapid clinical deterioration and high mortality seen in the original survival curves of Ross and Braunwald1 and confirmed by contemporary authors such as Otto et al.6 If operated upon, extreme-risk patients are considered to have a >50 % chance of death or permanent disability at 30 days. Balloon aortic valvuloplasty (BAV) was developed to address this group of patients who could not undergo SAVR. Although BAV could decrease the gradient and improve flow dynamics, it did not improve survival.
Cohort B of the PARTNER trial was the first and only trial in nonoperative patients to randomize TAVR using the valve against best medical therapy, which could include BAV.7 The primary endpoint was all-cause death. At 1 year there was a 20 % survival advantage to TAVR,7 and this advantage persisted for the 5-year life of the trial.8 The Extreme Risk Iliofemoral study of the CoreValve US Pivotal trial started after the reported 1-year endpoint of the PARTNER B trial; by then it was no longer considered reasonable to randomize against medical therapy due to the clinically significant advantage of TAVR. The US CoreValve Extreme Risk Pivotal Trial was a non-randomized registry comparing TAVR with the self-expanding valve against a performance goal based on the 95 % lower margin of survival for the medical arm of PARTNER B and five contemporary BAV series.9 TAVR with the self-expanding valve easily exceeded this goal at both 1 and 2 years.9,10 The Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System (REPRISE III) trial extreme-risk arm randomized TAVR with a mechanicallyexpandable valve against the self-expanding valve in a 2:1 fashion. The results were recently presented and found the Lotus™ Valve (Boston Scientific) to be safe and effective compared to commercially available self-expanding TAVRs (CoreValve and Evolut™, Medtronic).12
An issue that has become a challenge for valve teams in the management of extreme-risk patients is determining when a TAVR is futile and not appropriate in a patient with severe AS. There remains a sizable group of patients who die or lack improvement in quality of life (QoL) soon after TAVR.13 In this very elderly population (mean < 80 years) a number of factors in addition to traditional risk stratification need to be considered, including comorbidities, disability, frailty, and cognition, in order to assess the anticipated benefit of TAVR as well as the risk of an invasive procedure. Evaluation by a multidisciplinary heart valve team with broad areas of expertise is critical for assessing likely benefit from TAVR. Moreover, these complicated decisions should take place with clear communication around desired health outcomes on behalf of the patient and provider. Expectations need to be clear and realistic. For example, TAVR will not cure dementia and age-related cognitive decline.14 The approach should include a multidisciplinary evaluation. When there is concern regarding futility and expectations, additional members from the team are included in the consultation. Ultimately, the assessment to move forward with a TAVR must be a consensus opinion. The decision that treatment with TAVR is futile should include alternative plans to optimize the patient’s health state or, in some cases, discussions related to end-of-life care.
High-risk Patients
Cohort A of the PARTNER trial randomized patients with AS and a high risk of operative mortality to treatment with either SAVR or the SAPIEN (Edwards Lifesciences Corp) transcatheter valve. High risk was defined as either a Society of Thoracic Surgeons (STS) predicted risk of 30-day mortality (PROM) of at least 10 % or an expected risk of mortality of at least 15 % for SAVR as determined by two cardiac surgeons. The PARTNER A trial randomized patients with symptomatic severe AS as high risk for SAVR to TAVR versus SAVR.15 Survival at 1 year was equivalent and remained so for the 5-year term of the study.16 One question raised in the trial was the higher risk of stroke in TAVR compared with SAVR. The difference disappeared and strokes actually became numerically greater in SAVR by 2.5 years. Hemodynamic flow parameters measured by echo were equivalent but paravalvular leak (PVL) was substantially more common in TAVR than SAVR. Subsequently, the PVL rate in TAVR has substantially decreased as a result of more accurate annulus sizing using computer tomomgraphy angiogram better technique, and improved annular sealing using external skirts. QoL improved greatly and equivalently in both groups. The randomized CoreValve US Pivotal Trial High Risk study tested TAVR with a self-expanding valve against SAVR in a high-risk population.9 The trial had the provocative finding of actual superior survival for TAVR over SAVR at 1 and 2 years.9,17 At 3 years TAVR maintains a 6.4 % absolute survival advantage.18 In the trial the worry about stroke reversed as TAVR had less strokes than SAVR. Hemodynamic flow parameters by echo were statistically superior for TAVR over SAVR at all time points while PVL remained significantly higher in TAVR. Pacemaker implantation was higher with TAVR at 30 days and 1 year (19.8 % versus 7.1 %, p<0.001; 22.3 % versus 11.3 %, p<0.001). Like the PARTNER A trial, QoL increased dramatically and equally in both groups. The data led to commercial approval of both valves in the US and guideline recommendation for TAVR as an alternative to SAVR in this highrisk patient group.
Intermediate-risk Patients
A number of reports from Europe for intermediate-risk patient populations for TAVR versus SAVR in propensity-matched studies have shown equivalent survival that suggested equipoise in the intermediate-risk population.19–21 This lead to randomized trials for TAVR versus SAVR in the intermediate-risk population in the US. The PARTNER II trial cohort A randomized 2,032 patients for SAVR against TAVR with the secondgeneration balloon-expandable TAVR valve.22 The trial had separate arms for transfemoral (TF) and non-transfemoral (non-TF) access. The trial used an STS PROM of ≥4 but <10 to define intermediate risk. The primary endpoint was a non-hierarchical composite of all-cause mortality or disabling stroke at 2 years. The primary analysis was intent-to-treat but analysis of the as-treated and TF subgroups where prespecified. Over 1,000 patients were randomized into the TAVR and SAVR group with 789 and 716 available at 2 years for analysis in the TAVR and SAVR cohorts, respectively. The mean age was 81.5 and 81.7 years in the TAVR and SAVR groups, respectively, with corresponding mean STS score of 5.8 % in both arms. Anesthesia time, procedure time, intensive care unit time, and length of stay were all significantly shorter in the TAVR group. The primary endpoint in the intent-to-treat group of all-cause mortality or disabling stroke for TAVR versus SAVR at 1, 12 and 24 months was 6.1 %, 14.5 %, and 19.3 % versus 8.0 %, 16.4 %, and 21 %, respectively (p=0.253), easily reaching non-inferiority at p=0.001. The as-treated numbers were similar with a p=0.180. Subgroup analysis suggested that non-TF access trended towards favoring SAVR at p=0.06. When looking at the prespecified TF-only intent-to-treat group for the primary endpoint, TAVR versus SAVR at 1, 12 and 24 months was 4.9 %, 12.3 %, and 16.8 % versus 7.7 %, 15.9 %, and 20.4 %, respectively, (p=0.05). This reached statistical superiority for TAVR in the as-treated TF group with TAVR versus SAVR at 2 years (16.3 % versus 20.0 %, p=0.04). Major vascular complications were more common in TAVR versus SAVR (7.9 % versus 5.0 %, p=0.006), while life-threatening and disabling bleeding (10.4 % versus 43.4 %, p <0.001), acute kidney injury (1.3 % versus 3.1 %, p=0.02), and new atrial fibrillation (9.1 % versus 26.4 %, p< 0.001) were all significantly more common in SAVR. Aortic valve area by echo was significantly better in TAVR at all time points. The trial supported the use of the second-generation balloonexpandable TAVR valve in the intermediate-risk population.
The Surgical Replacement and Transcatheter Aortic Valve Implantation trial (SURTAVI) trial was a prospective randomized trial of TAVR with the self-expanding valve versus SAVR in an intermediate-risk population.23 The trial randomized TAVR to SAVR 1:1 with each group having subgroups of patients requiring revascularization or not. Intermediate risk in the trial is defined as an estimated 30-day surgical mortality of 3–15 % according to the criteria of the STS PROM. The primary endpoint was all-cause mortality or disabling stroke at 24 months. A total of 1,746 patients underwent randomization at 87 centers. Of these patients, 1,660 underwent an attempted TAVR or surgical procedure. The mean (± SD) age of the patients was 79.8 ± 6.2 years, and all were at intermediate-risk for surgery (STS PROM 4.5 ± 1.6 %). At 24 months the estimated incidence of the primary endpoint was 12.6 % in the TAVR group and 14.0 % in the surgery group (95 % credible interval [Bayesian analysis] for difference, -5.2–2.3 %; posterior probability of non-inferiority >0.999). Surgery was associated with higher rates of acute kidney injury, atrial fibrillation, and transfusion requirements, whereas TAVR had higher rates of residual aortic regurgitation and need for pacemaker implantation. TAVR resulted in lower mean gradients and larger aortic valve areas compared with surgery. Structural valve deterioration at 24 months did not occur in either group. The conclusion was TAVR was a non-inferior alternative to surgery in patients with severe AS at intermediate surgical risk, with a different pattern of adverse events associated with each procedure.
The Safety and Performance Study of the Edwards SAPIEN 3 Transcatheter Heart Valve (SAPIEN3) trial was a non-randomized registry for TAVR using the third-generation balloon-expandable valve in an intermediate-risk population defined as an STS score of 4–8 %.24 A prespecified comparison to the surgical arm of PARTNER IIA was planned using a prespecified propensity score analysis with patient level data. The trial recruited 1,078 patients who were divided into TF and non-TF access (transapical and transaortic). The design was a non-inferiority primary endpoint composite of all-cause mortality, all stroke, and moderate or greater PVL with a hierarchical prespecified superiority test if non-inferiority was met. The mean age for TAVR and SAVR was 81.9 and 81.6 years with a mean STS of 5.2 and 5.4, respectively. The trial reached non-inferiority for the composite primary endpoint at p<0.001 followed by superiority at p<0.001. Looking at the individual components of the endpoint, TAVR reached superiority for mortality (p<0.001) and stroke (p=0.004) but SAVR was superior for PVL (p=0.0149). All-cause death and all stroke for TAVR versus SAVR at 30 days and 1 year were 1.1 % and 7.4 % versus 4.0 % and 13.0 %; 2.7 % and 4.6 % versus 6.1 % and 8.2 %, respectively, showing an extremely low mortality and stroke rate for TAVR in this elderly population. Moderate or greater PVL in the TAVR cohort in this trial was only 1.5 % at 1 year.
Both TAVR platforms are currently Food and Drug Administration approved in the US for the use in treating intermediate-risk patients with severe symptomatic AS. All patients who meet this profile should be evaluated by a heart team and offered TAVR as an alternative to SAVR with an understanding of the advantages and disadvantages of each modality.
Low-risk Patients
The Nordic Aortic Valve Intervention (NOTION) trial compared TAVR with SAVR in predominantly low-risk patients.25 Patients ≥70 years with severe AS and no significant coronary artery disease were randomized 1:1 to TAVR using a self-expanding bioprosthesis versus SAVR. The primary outcome was the composite rate of death from any cause, stroke, or MI at 1 year. A total of 280 patients were randomized with a mean age of 79.1 years, and 81.8 % were considered low-risk patients. In the intention-to-treat population, no significant difference in the primary endpoint was found (13.1 % versus 16.3 %, p=0.43 for superiority). The result did not change in the as-treated population. No difference in the rate of cardiovascular death or prosthesis reintervention was found. Compared with SAVR, TAVR had more conduction abnormalities requiring pacemaker implantation, larger improvement in effective orifice area, more total aortic valve regurgitation, and higher New York Heart Association functional class at 1 year. SAVR had more major or life-threatening bleeding, cardiogenic shock, acute kidney injury (stage II or III), and new-onset or worsening atrial fibrillation at 30 days compared with TAVR. This study had several limitations as it was a small trial and not fully reflective of the population of low-surgicalrisk patients with AS.
Low-risk patients are determined by the heart team to have <3 % surgical risk or an STS PROM score of <4 %. Two low-risk prospective randomized trials are currently enrolling in the US: the PARTNER III trial of the balloon-expandable valve (The Safety and Effectiveness of the SAPIEN 3 Transcatheter Heart Valve in Low-risk Patients with Aortic Stenosis, NCT02675114) and the Evolut R low-risk randomized trial for the selfexpanding valve (Medtronic Transcatheter Aortic Valve Replacement in Low-risk Patients, NCT02701283). Low-risk patients in the US should be referred to a center with a heart team and evaluated for participation in a randomized clinic trial comparing TAVR and SAVR. Alternatively, in 2017, whether these patients should be treated with SAVR as TAVR has not yet been completely evaluated. Many who are evaluated as low risk via screening may have a bicuspid aortic valve. These patients are currently excluded from the current protocols.
For TAVR to be considered as an alternative to SAVR in low-risk patients it would need to show equivalent or better mortality, morbidity, hemodynamics, QoL, patient acceptance, and durability. The available data would support equivalent or better mortality, hemodynamics, and QoL. A continuing challenge and limitation of TAVR remains conduction disturbances requiring a permanent pacemaker. As younger and lowerrisk patients are treated this will become less acceptable. Although little directly-measured data on patient acceptance exists, valve clinic practitioners understand patients overwhelmingly desire TAVR as their treatment choice. The morbidity of TAVR has improved substantially. The data on stroke now suggest it is higher in SAVR than TAVR and PVL has improved substantially through valve design in the short period that TAVR has existed. SAVR continues to show higher rates of severe bleeding, AKI, and atrial fibrillation in multiple randomized trials.
Durability
Long-term valve durability concerns have remained a limitation in the expansion of TAVR to lower-risk patients. The 5-year follow-up data from PARTNER16,26 are reassuring in that no episodes of structural deterioration requiring replacement were observed, and valve hemodynamics (gradient and effective orifice areas) remained stable. Furthermore, biomechanical stresses are likely lower in transcatheter patients than surgical patients due to the lower residual mean gradients and the effective orifice area in transcatheter patients. Regardless, additional follow-up data in transcatheter valves will be required in the future to declare equivalence with the most durable surgical bioprosthetic valves.27 The transcatheter valves currently used in practice appear equivalent in the short to intermediate term. Perhaps the uncertainty of long-term durability becomes less concerning with the possibility of transcatheter valve-in-valve therapy to extend the duration of nonsurgical valve treatment.
Conclusion
Current data suggest TAVR is not just an alternative but the best choice for nonoperative patients with severe symptomatic AS in both extreme- and high-risk patients with anatomy suitable for TAVR. In addition, TAVR is a reasonable alternative in the intermediate-risk group with appropriate anatomy. Two low-risk randomized trials have started in the US (PARTNER III and Evolut low-risk randomized trials) to determine the feasibility of TAVR as an acceptable alternative to SAVR in low-risk patients with severe AS. In 2017, all patients with severe symptomatic AS should be referred to a valve center that can offer the entire spectrum of treatment modalities including TAVR and SAVR as part of routine care or the opportunity to participate in a clinical trial to answer some of the remaining questions surrounding TAVR.