Transcatheter aortic valve replacement (TAVR) is a major advancement in the treatment arsenal for elderly patients with severe aortic stenosis with intermediate-to-high surgical risk. According to recent trial studies, TAVR carries similar or superior clinical outcomes compared to surgical aortic valve replacement (SAVR) even in a population with a low surgical risk, thereby, encompassing the younger and low-risk population as well.1–3 Consequently, the number of TAVR procedures have increased exponentially over the years.
Despite the advancements and introduction of new generation TAVR devices, cerebrovascular events (CVE) remain one of the most serious complications. Interestingly, the incidence of clinical CVE post-TAVR is less than 5% and the incidence of silent lesions evaluated by diffusion-weighted MRI (DW-MRI) is 60–90%, irrespective of the type of device or access site.4–6 These silent lesions are associated with increased risk of stroke, dementia, and long-term cognitive decline as well.7 A study conducted by Lansky et al. further cemented the findings by demonstrating that 94% of patients who underwent DW-MRI had brain lesions and 41% showed a cognitive decline from baseline to 30-day follow-up when assessed using Montreal Cognitive Assessment.8 Although there is no difference in rates of stroke between TAVR and SAVR at 2-year follow-up, TAVR is associated with a heightened risk of neurological complications.9 Moreover, Huded et al. demonstrated that 30-day stroke risk after TAVR remains stable over 5 years.10 Major cerebral embolic events during TAVR have been recognized as an independent morbidity and mortality predictor, strongly affecting quality of life, blunting the cognitive function and day-to-day abilities.11,12 A recent nationwide readmission database study showed that patients with stroke after TAVR with embolic protection devices (EPD) use had significantly lower in-hospital mortality when compared to patients with stroke after TAVR without EPD use.13 In addition, our group reported that EPD use during TAVR may be associated with reduced severity of stroke despite there being no significant difference in overall stroke incidence compared with not using cerebral embolic protection (CEP).14
Thus, EPD have been designed and developed to protect from CVE by either filtering or deflecting potential cerebral emboli during TAVR. Some devices have received a CE mark or Food and Drug Administration (FDA) approval and are used in clinical practice globally. In our review, we discuss the risk factors and etiology of CVE and elaborate on the different types of EPDs currently available along with their respective clinical data.
Mechanism of Cerebrovascular Events
The TAVR procedure involves a retrograde approach with advancement of large-bore delivery catheters through aortic arch and direct maneuvering of the calcified aortic valve. Scraping of the aortic plaques is witnessed in >50% of percutaneous cardiac procedures and is more often seen with larger catheter systems.15,16 Consequently, dislodgement of debris from either the varying degrees of atheroma in the aortic arch or from the aortic valve itself can result in an embolic stroke. Additionally, the squashing of the native calcified leaflets by the implantation of the prosthetic valve can further heighten the risk of embolic stroke. Risk factors for peri-procedural CVE are categorized under patient- and procedure-related factors. Patient-related factors include increasing age and degree of ascending aortic atheroma burden. Both are independently associated with elevated CVE risk.17 Most of the procedural-related factors are attributed to the micro-debris that occurs after guidewire movements, dilation and insertion or balloon removal from the valve, or valve implantation itself.6 The catheters, wires and delivery systems used during TAVR are pro-thrombotic and could be the source of air emboli, resulting in an increased stroke risk.18 Increased burden of embolic debris in great vessels during TAVR is also associated with prosthetic valve cover index – a measure of the size of prosthetic valve relative to the native annulus size – and the use of balloon-expandable prostheses.19
CVE after TAVR can be categorized under three phases – early, delayed, and late.20 Early-phase CVE is seen in high-risk patients where the stroke is related to above mentioned procedure-related factors where all the CVE occur within 24 hours of TAVR. However, in some trials and guidelines, stroke within 72 hours of the procedure is defined as procedural stroke.21 Many brain imaging studies using DW-MRI before and during the first few days after TAVR evaluated the early onset of stroke. Up to 84% of the patients had new lesions on DW-MRI after TAVR.4,6,17,22,23 Moreover, balloon aortic valvuloplasty procedure and rapid pacing can result in diminished blood flow to the watershed areas of the brain circulation, which in turn can minimize the embolized debris washout furthering the CVE risk burden.24,25 The delayed phase of the stroke is seen in increased risk interval between days 3 and 30. The major reason behind the delayed CVE is thromboembolism as a result of thrombogenicity. New onset AF (NOAF) after TAVR is known to be an independent predictor of delayed stroke.26,27 Many studies have established the association of NOAF and stroke after TAVR. Nuis et al. confirmed a 4.4-fold increase in the stroke risk in patients with NOAF compared to patients without NOAF after TAVR.28 Amat-Santos et al. also showed a 40% stroke rate in NOAF patients without anticoagulation in contrast to a 2.9% stroke rate in immediately anticoagulated patients.26 Moreover, an extended time for endothelialization of the artificial nitinol surfaces or hypo-attenuated leaflet thickening (HALT) of TAVR valves can be another risk factor for delayed stroke.28,29 The late phase of stroke is seen in patients with a late hazard interval and is commonly patient- and disease-related. The constellation of comorbidities, such as hypertension, dyslipidemia, diabetes, obesity, nicotine addiction, and older age, are some atherogenic risk factors which can be seen in patients with aortic stenosis leading to a higher risk of CVE.30,31
The rate of stroke is different among the clinical trials. While the rate of stroke was noticed to be considerably higher at 30 days in patients at high operative risk (5.5%) and inoperable patients (6.7%) when compared to a SAVR cohort (2.4%) or patients undergoing standard medical therapy (1.7%) in the PARTNER I trial, the PARTNER III trial demonstrated a significantly lower stroke rate at 30 days in the TAVR cohort (0.6%) when compared to patients undergoing SAVR (2.4%).2,32 This difference could be potentially explained by more operator experience, device development or patient selection.33 Further, in the PARTNER IA and IB trials, neurological examination was not systematically performed and this may make the stroke ascertainment unreliable.34 It is important to note that in PARTNER IIA where neurological examination was systematically performed, the SAVR stroke rate was reportedly higher (6.1%) compared to 2.4% in PARTNER 1A.32,35 It is also important to realize that most studies report major, disabling stroke, or minor, non-disabling stroke, with transient ischemic attack (TIA) being seldom reported with the incidence of major stroke being 58%, minor stroke 26%, and TIA 16%.36 However, this variation could be a consequence of lack of optimal and systematic neurological evaluation to detect minor strokes or TIA and can be further explained by study designs, the definition of stroke used, diagnostic evaluation strategy and site-specific factors.37–39 The quality of neurological assessment is another factor that could affect the rate of stroke after TAVR. Some studies have also established that the stroke rate and identification of silent brain infarction is greater if the neurological assessment is performed by a neurology fellow or a neurologist.34,40
In general, the greater the manipulation of the stenotic and calcified aortic valve and annulus, the larger the risk of CVE. Nonetheless, it is important to note that CVE post-TAVR is substantially unpredictable from baseline patient demographic data and comorbidities.41 Further, more procedural experience does not diminish the risk of CVE after TAVR.42
Etiology
Understanding the origin of embolic materials is crucial in diminishing the peri-procedural CVE incidence during TAVR. Debris captured from cerebrovascular circulation of 81 patients were assessed histopathologically which revealed a wide array of tissue types. Where 74% patients were witnessed to have fibrin- and thrombus-derived debris, tissue-derived debris was seen in 63% patients. Endothelial and vessel wall tissue was retrieved in 48% cases and valve-derived debris was seen in 33% patients.18
Many clinical trials have also been conducted to assess the histopathology of the debris captured from the EPDs which also revealed thrombus, valve tissue, calcification, vessel wall, and foreign bodies.41 The lower limit of diameter of debris has also been assessed histologically based on the pore sizes of EPDs.43 The particle size of the arrested debris ranges from 150 to ≥2,000 µm.43 In the randomized trial conducted by Kapadia et al., almost more than 80% of the captured debris size varied from 150 to 500 µm and <5% debris of >1,000 µm size.40 In another study by Van Mieghem et al., the captured debris particle size was noted to vary from 150 to 4,000 µm.44 It has also been insisted that embolic materials captured by EPDs among different transcatheter heart valves are different.45,46 Nonetheless, debris was captured by EPDs in the majority of the patients. Even for valve-in-valve procedures, the type of debris captured by filter was similar to those captured during TAVR for native valves.47
Neurological Protection Devices
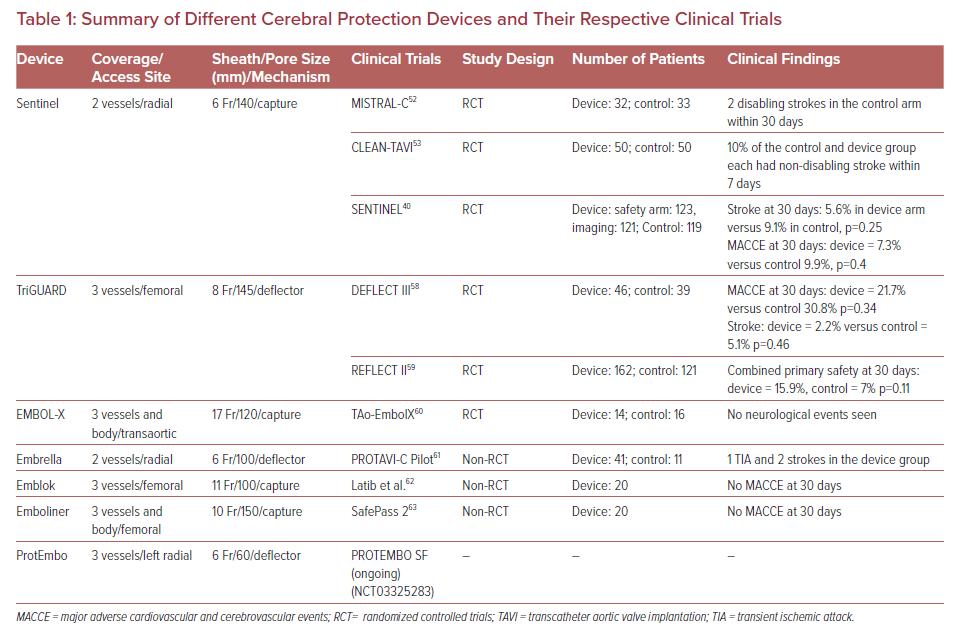
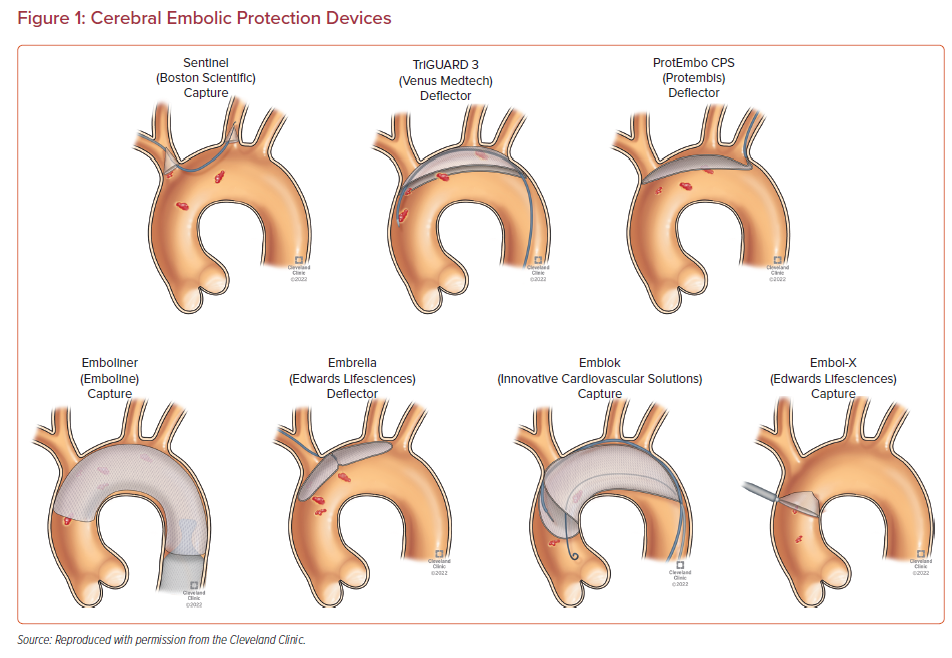
Multiple devices have been developed to prevent embolizing events during TAVR procedure. They are designed to capture or deflect the emboli en route to the brain during TAVR. These EPDs are usually stationed across the supra-aortic vessels origin prior to the furtherance of the TAVR system across the aortic valve and are recovered at the end of the procedure.48 The most suitable devices cover the ostia of three large branches of the aortic arch, carry filter capabilities, facilitate procedural stability, and safeguard the delicate and calcified wall of the aortic arch. Given the full perimeter coverage, it ensures an adequate brain protection.49 The different types of the EPDs and their respective clinical trials along with their pictorial descriptions are summarized in Table 1 and Figure 1, respectively.
Sentinel
Sentinel cerebral protection device (Boston Scientific) is the most studied system and was given a CE mark in 2013 and FDA approval in 2017.49 It is designed to capture debris dislodged during TAVR rather than deflecting to the peripheral circulation. The Sentinel device consists of a dual filter system (left common carotid and brachiocephalic artery) within a 6 Fr delivery catheter system which is accessed percutaneously via the right radial/brachial artery using a 0.014-inch guidewire. The proximal filter (diameter 9–15 mm) is delivered in the brachiocephalic artery and the distal filter (diameter 6.5–10 mm) in the left common carotid artery prior to TAVR and is withdrawn into the catheter and removed after TAVR.49 The Sentinel device has only one available size. Therefore, complete sealing might not be feasible in different aortic anatomies.50 It is crucial to note that the left vertebral artery remains unprotected. To overcome this, an additional Wirion filter (Allium Medical) defending the left vertebral artery can also be used for complete neurological protection during TAVR.51 The device deployment takes less than 10 minutes in 91% of the patients undergoing TAVR.49
There are three large randomized controlled trials (RCTs) for the Sentinel device. The MISTRAL-C trial was the first RCT to evaluate the efficacy and safety of this device.52 It was a multicenter, double-blinded study of 65 patients randomized 1:1 to transfemoral TAVR with and without the Sentinel device. The aim was to compare the number of new cerebral lesions evaluated by DW-MRI and the assessment of neurocognitive functions before and 5 days after TAVR. The filter showed debris in all patients in the EPD group. The percentage of patients with new cerebral lesions (the primary endpoint) was numerically lower in the EPD group, along with a lower volume of brain lesions as detected by DW-MRI. Further, neurocognitive deterioration was less frequent in the EPD group (4% versus 27%; p=0.017) and there was a considerable decrement in patients with more than 10 cerebral lesions (20% versus 0%; p=0.03).52
The CLEAN-TAVI trial randomized 100 patients and assessed new cerebral lesions investigated by DW-MRI 2 days after TAVR.53 The EPD group had a decrement in the new-onset brain lesions in protected territories (4 versus 10; p≤0.001) as well as throughout the brain (8 versus 16; p=0.002). The median total new lesion volume was also smaller in the EPD group (466 mm3 versus 800 mm3; p=0.02).
The third trial – SENTINEL – was the largest randomized study, involving 363 patients who underwent TAVR from 19 centers in Germany and the US. Patients were randomized 1:1:1 in safety arm with the device and two imaging groups which randomly underwent TAVR with (device arm) and without (control arm) the Sentinel device. The device positioning was successful in all the patients. Debris was captured in filters in 99% of the patients. The new lesion volume (primary efficacy endpoint) was comparable between the groups (102.8 mm3 versus 178 mm3; p=0.33). The primary safety endpoint of major adverse cardiovascular and cerebrovascular events (MACCE) at 30 days was 7.3% in the device arm and 9.9% in control arm (p=0.4). The rate of stroke was lower in the EPD group without statistical significance (5.6% versus 9.1%; p=0.25).40 Although this trial did not meet the primary imaging endpoint, the Sentinel EPD still received FDA approval because of the high rate of debris captured by the filter. Moreover, a post hoc analysis also revealed a reduction of new lesion volume on brain MRI.54
SENTINEL-LIR was another trial looking into frequency of embolic capture by the Sentinel device in a total of 50 low-risk TAVR cases. It reflected that embolic debris capture in low-to-intermediate risk TAVR patients were similar to the high-risk patients undergoing TAVR and potentially having similar embolic risk as the high-risk cohort.55
Another trial, PROTECTED TAVR (NCT04149535), is ongoing and will assess the efficacy of the Setinel device in 3,000 patients with stroke 72 hours post-TAVR or discharge (whichever comes first) as the current primary end outcome.
TriGUARD
The TriGUARD device (Keystone Heart) is the second most studied device. It is the only available device that is designed to encompass all the arteries in the aortic arch. It is a deflector device rejecting the emboli during TAVR towards the descending aorta. The TriGUARD is advanced through a 9 Fr arterial sheath that is placed into the contralateral femoral artery and is deployed to cover the ostia of all three aortic arch branches.48
Two RCTs, DEFELCT I and DEFLECT II have been conducted for this device system showing that first- and second-generation devices are safe and effective.56,57 DEFLECT III was the first multicenter, randomized controlled study to evaluate the safety and efficacy of TriGUARD in TAVR.58 It was conducted at 13 sites across five countries in Europe and Israel and the study was completed in March 2015. Patients were randomized into an EPD group (n=46) and an unprotected group (n=39). Complete coverage was witnessed in 89% patients. The MACCE and stroke rate at 30 days was lower in the device arm without statistical significance (MACCE at 30 days: 21.7% versus 30.8%, p=0.34; stroke at 30 days: 2.2% versus 5.1%, p=0.46). There was a non-significant decrement in neurological impairment (3.1% versus 15.4%, p=0.16) and lesions volume.
The new generation TriGUARD 3 has been investigated in the REFLECT II trial and the results were published in 2021.59 TAVR patients were randomized to a device arm (n=162) and a control arm (n=121). The composite primary safety endpoint at 30 days (15.9% versus 7%, p=0.011) and median total new lesion volume (215.4 mm3 versus 188.1 mm3; p=0.4) assessed by DW-MRI at 2–5 days were similar in both groups.
Other Devices
Other EPDs include EMBOL-X, Embrella, Emblok, Emboliner, and ProtEmbo.
EMBOL-X device (Edwards Lifesciences) is used in open-heart surgery at the aortic cannulation site.60 The efficacy and safety was evaluated in an RCT involving 30 patients who underwent transaortic TAVR (device arm n=14, control arm n=16). The mean new brain lesion was evaluated at 1 week by DW-MRI. No neurological event was seen in either group on follow-up. Moreover, the device group showed numerically smaller lesion volume than the control group without statistical significance (88 versus 168 mm3, p=0.27).
The Embrella device (Edwards Lifesciences) was developed to deflect embolic material during TAVR. It consists of an oval shaped nitinol frame covered with a porous membrane which is designed to protect all three cerebral vessels. The device is delivered via right radial/brachial approach through a 6 Fr sheath. The PROTAVI-C trial studied this device involving 52 patients (41 with the device and 11 without).61 It revealed that the use of the Embrella device was associated with considerably higher amounts of high-intensity transient signals (surrogate of micro-embolization evaluated using transcranial Doppler) compared to the control group (632 versus 279, p≤0.001). Total lesion volume was significantly less in the device arm (30 mm3 versus 50 mm3; p=0.003).20–49,50-68
The Emblok device (Innovative Cardiovascular Solutions) has been developed to be positioned in the ascending aorta and aortic arch via femoral artery using a 11 Fr sheath, thereby providing a circumferential aortic protection from embolic materials. The first-in-human trial for this device involved 20 patients and demonstrated no MACCE at 30-day follow-up.62 DW-MRI post-procedure showed 95% of patients had new ischemic brain lesions, but the medial new total lesion volume was 199.9 mm3 (interquartile range: 83.9–447.5 mm3).
The Emboliner (Emboline) device system is advanced from a 9 Fr transfemoral sheath used for the 6 Fr pigtail catheter for TAVR. It is engineered to protect all three cerebral vessels and the whole body. Early results from the SafePass 2 trial were presented in Transcatheter Cardiovascular Therapeutics 2019 reflecting no adverse events at 30 days with 100% procedural success.63
ProtEmbo device system (Protembi) defends all the three cerebral vessels and has the smallest filter pores. It is delivered via a 6 Fr sheath through left radial/brachial artery. The PROTEMBO SF trial is ongoing to assess the safety and efficacy of the ProEmbo device (NCT03325283).
Several new EPDs are in development. Point-Guard EPD is one such device which promises to provide a complete cerebral protection by covering all supra-aortic arteries via deflection, capture or removal of the debris. It consists of a flexible nitinol frame with filter mesh wrapped around its perimeter. It also has a supporting extension at the distal end. By sealing and conforming in compliance with the aortic arch anatomy, it addresses the challenge of unfiltered blood flowing to the brain and travelling around other partial protective devices or devices with non-sealing edges. Transverse Medical commenced the Point Guard CENTER trial in 2018 and is set to be carried out at multiple institutions in the European Union. The endpoints are focused on performance and safety of the device.48,49
Embolisher is another investigational EPD with a deflector mechanism and a contralateral transfemoral access. By positioning in the aortic arch, it covers all supra-aortic arteries and delivers a complete cerebral protection.48
Filterlex EPD (Filerlex Medical) is also under development and carries a deflector mechanism with an ipsilateral transfemoral access. Positioning is in the aortic arch and descending aorta, it promises to provide full cerebral as well as body protection.48
Many trials and studies have demonstrated the safety of EPDs. A meta-analysis conducted by Zahid et al. demonstrated significant minimization of MACCE, mortality, and stroke without considerable differences in acute kidney injury, bleeding or any vascular complications in patients undergoing TAVR with the use of EPD as compared to a TAVR cohort without EPD usage.64 Similarly, other meta-analyses by Ndunda et al., Shimamura et al., and Mohananey et al. Have also established a lower risk of stroke with EPD use in TAVR.65–67 In another meta-analysis by Bagur et al., EPD use during TAVR was associated with smaller silent ischemic volume per lesion and smaller total volume of silent ischemic lesions without any considerable differences based on clinical stroke.68 However, the efficacy is still lacking in the literature due to the small incidence of CVE after TAVR. It requires patients to undergo repeated DW-MRI which can be cumbersome for elderly patients. Some may have baseline neurological deficits which could make the stroke evaluation taxing. Recent large-scale observational studies have also reported that EPD use for TAVR was not significantly associated with a lower rate of stroke following TAVR.69–71 Butala et al. conducted a primary analysis using an instrumental variable model using the Transcatheter Valve Therapy Registry showing no relation between EPD usage during TAVR and in-hospital stroke (adjusted RR 0.90, 95% CI [0.68–1.13]); absolute risk difference, −0.15%; 95% CI [−0.49, 0.20]). Despite a negative primary endpoint, a secondary analysis using a propensity score model was performed which reflected 18% lower odds of in-hospital stroke (adjusted OR 0.82, 95% CI [0.69–0.97]) with absolute risk difference of −0.28%; 95% CI [−0.52, −0.03]) with the use of EPDs. The results were generally uniform across the other secondary endpoints and subgroup analyses.69
To answer most of these shortcomings and variations, if not all, the ongoing PROTECTED-TAVR trial (NCT04149535) will assess the efficacy of the Sentinel device in 3,000 patients. The routine use of EPD will be in action if this trial displays positive efficacy results. However, it comes with some limitations. The efficacy of the device systems with complete protection of all three cerebral vessels and descending aorta will still have to be verified as the Sentinel device does not defend the left vertebral artery and descending aorta. Cost effectiveness will need to be entrenched as overall CVE post-TAVR is small and clinical detected stroke is seldom witnessed. Another major trial – BHF-PROTECT TAVI (ISRCTN16665769) – is under way, assessing the use of Sentinel EPD in patients undergoing TAVR. The primary outcome is all-cause stroke through 72 hours post-TAVR or discharge, whichever comes first. The sample size is aimed at 7,730 patients with a planned completion in 2025.72
In future, further large-scale randomized clinical studies to examine the efficacy of CEP with clinically relevant endpoints is needed. Additionally, identifying a way to predict a high-risk population for stroke can be useful to target this population for CEP. Cost analysis of CEP is another integral area for further research.
Conclusion
CVE is one of the most serious and feared complications associated with TAVR despite the technological advancements and operator experience. As most cases are procedure-related embolization, EPDs have an excellent potential to prevent acute embolism. However, it should be noted that the efficacy of EPD on the reduction of CVE has not been proved with any type of device. Further adequately powered RCTs are needed to establish the optimal role of EPDs in TAVR procedure.