Primary cardiac tumors are extremely rare, having an incidence of 0.001–0.28% in an autopsy series.1 By contrast, metastatic tumors to the heart are some 30-fold more common.2 The most frequent clinical manifestations associated with primary cardiac lymphoma (PCL) are pericardial effusion, heart failure, and atrioventricular block.3 Due, in part, to late recognition, as well as a lack of a standard approach to treatment, PCL can have a grim prognosis, with survival of less than 1 month. With proper diagnosis and management, survival can be extended to 5 years.4 However, diagnosis can often be challenging, requiring surgical biopsy for definitive diagnosis. We present a case where multimodality cardiac imaging was used in order to aid in and expedite diagnosis.
Case
A 76-year-old man with no significant past medical history presented to the emergency department for shortness of breath that had worsened over the 1 week prior to presentation, especially on exertion. He also reported abdominal discomfort and a 6.8 kg (15 lb) weight loss over the previous month. He denied any known history of heart disease and did not have any chest discomfort, denied any fevers or chills, and denied any previous smoking history.
On initial examination, the patient was afebrile, had a normal heart rate, and had a normal O2 saturation of 99% on room air. He was tachypneic, with a respiratory rate of 28 breaths/min, and mildly hypertensive, with a blood pressure of 149/89 mmHg. He was noted to be in no acute distress.
Physical examination revealed a jugular venous pressure of 7 cm above the sternal angle with positive hepatojugular reflux and borderline Kussmaul’s sign without significant collapse of the neck veins with inspiration. The heart sounds were normal with normal S1 and S2 without murmurs, rubs, or gallops. The patient had bilaterally diminished breath sounds but no rales, rhonchi, or wheezing. He had grade 1 lower extremity pitting edema bilaterally.
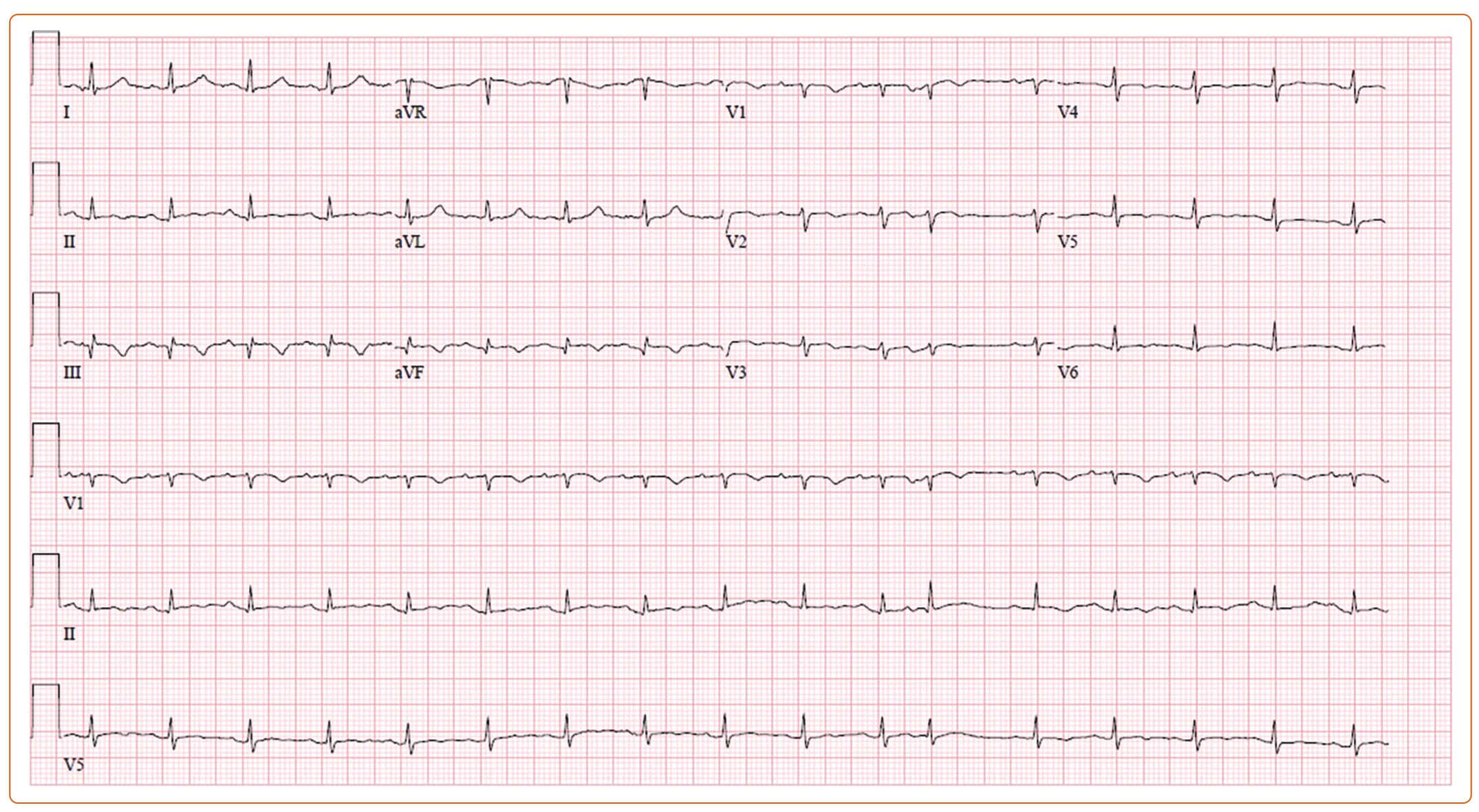
Initial laboratory results were unrevealing, other than an N-terminal pro B-type natriuretic peptide elevated to 2,200 pg/ml. EKG revealed a low-voltage QRS with sinus tachycardia and T wave inversions inferiorly without ST abnormalities or electrical alternans (Figure 1). A CT of the chest with contrast was performed, and a large pericardial effusion with small bilateral pleural effusions was found with prominence/thickening of the anterior right ventricular (RV) wall noted.
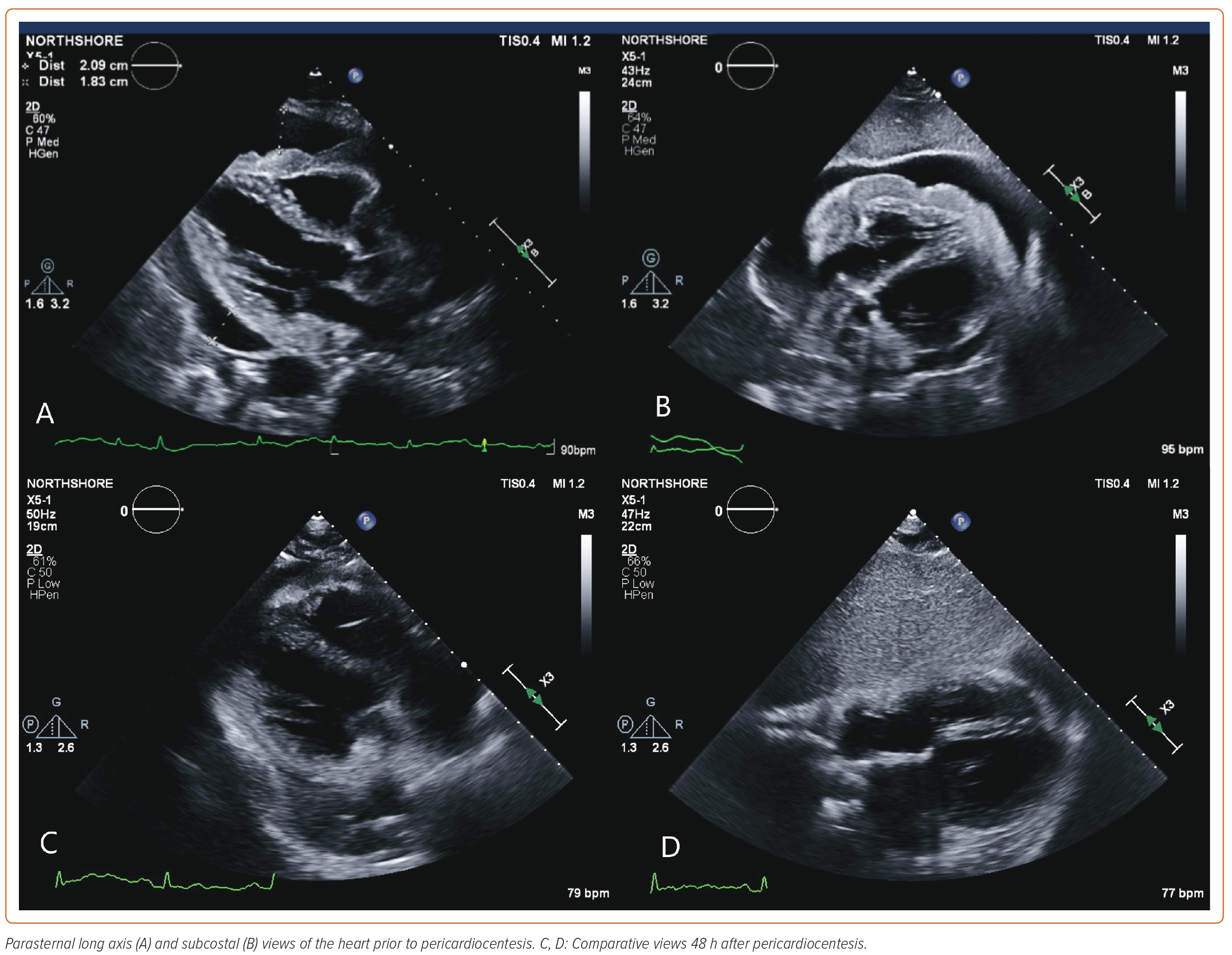
A subsequent urgent transthoracic echocardiogram (TTE) revealed a normal left ventricular ejection fraction (LVEF) of 61% without regional wall motion abnormalities, but a large circumferential pericardial effusion and a homogeneous mass on the entire anterior RV wall were noted (Figures 2A and 2B). There was right atrial diastolic collapse and borderline respirophasic variation of mitral and tricuspid inflows with a distended, non-collapsing inferior vena cava, which were concerning signs of impending tamponade. An urgent pericardiocentesis was performed, with 560 ml sanguineous fluid removed and sent for cytology and placement of a pericardial drain. Pericardial pressure was reduced from 43 to 12 mmHg with pericardiocentesis. The patient’s symptom of shortness of breath was almost completely resolved with pericardiocentesis.
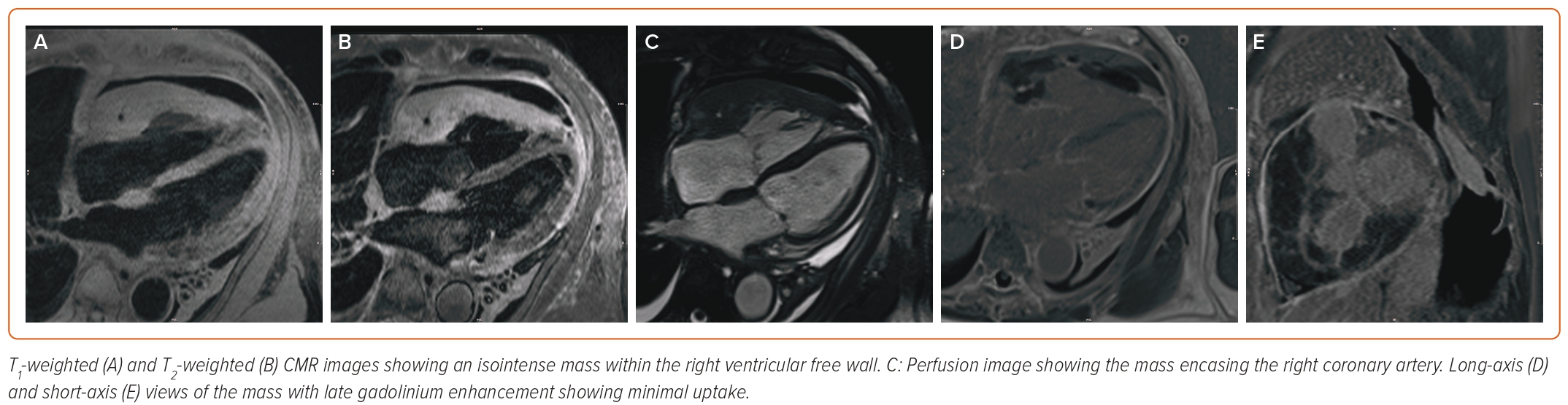
Repeat TTE performed 48 h after drain placement revealed a small residual pericardial effusion with revisualization of the RV mass (Figures 2C and 2D). While awaiting final cytology results, the patient underwent cardiac MRI (CMR) to further characterize his cardiac mass; the results are shown in Figure 3. The myocardial mass showed invasion of the myocardium with isointense T1 and T2 signals and modest early and late gadolinium contrast enhancement, which were concerning signs of cardiac lymphoma.
Cytology ultimately demonstrated neoplastic proliferation of large, atypical lymphoid cells characterized by a high nucleus-to-cytoplasm ratio and nuclei with coarse hyperchromatic chromatin and discernible nucleoli. Frequent apoptotic tumor cells were also noted. Immunohistochemical staining for CD3, CD5, CD10, CD20, Bcl-2, Bcl-6, Mum-1, C-myc, Ki-67, and TdT was performed on cell block sections. Tumor cells were positive for CD10, CD20, and Bcl-6. Only weak staining for Mum-1 and C-myc was seen in 30–40% of tumor cells. Tumor cells were negative for Bcl-2 and TdT. The stains for CD3 and CD5 highlight non-neoplastic T cells. The tumor cells also showed a high proliferative index based on Ki-67 staining (~80–90%). Overall, the findings are those of a high-grade B-cell lymphoma with a germinal central-like phenotype.
CT of the chest, abdomen, and pelvis, followed by whole-body PET, found no evidence of other sites of malignancy, and the patient was diagnosed with high-grade primary cardiac B-cell lymphoma. Hematology was consulted and the patient was started on R-CHOP (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 2 mg, and prednisone 100 mg) therapy while an inpatient. The plan was for six 21-day cycles of chemotherapy with curative intent. Repeat CMR after three cycles demonstrated nearly complete resolution of the pericardial/myocardial mass. The patient was able to undergo the full six cycles of treatment and tolerated it well, with a repeat CT scan demonstrating no evidence of disease.
Unfortunately, the patient developed anthracycline toxicity approximately 12 months after finishing treatment with progressive worsening of his LVEF to <20%, resulting in hospitalization with cardiogenic shock. After a course of mechanical and inotropic circulatory support, the patient was discharged with home hospice for end-stage heart failure in alignment with his goals of care.
Discussion
Primary cardiac lymphomas are rare neoplasms, comprising 1.3% of all cardiac tumors, with an overall incidence of 0.001–0.28%.1 Given their rarity, these are mostly described in the literature as single case reports.
The present case demonstrates the utility of CMR in the diagnosis of undifferentiated cardiac masses. Because PCLs are often aggressive with very high rates of mortality, prompt diagnosis is of utmost importance. In our case, CMR was able to diagnose likely cardiac lymphoma before cytology results returned, allowing us to consult oncology and start the process of preparing the patient for chemotherapy before a tissue biopsy had returned. Although in our case cytology could be obtained from pericardiocentesis, in cases where thoracic surgery may be required for pericardial biopsy CMR would be even more helpful to expedite care while surgery and pathology results are pending. In evaluating cardiac masses by CMR, physical characteristics, such as location, size, and invasion of tissues, is first evaluated. The mass can then be evaluated using T1- and T2-weighted imaging, as well as late gadolinium enhancement.5 Cardiac lymphoma is characterized, as in our case, by isointense T1 and T2 mapping with limited to no late gadolinium enhancement, while demonstrating a mass that invades fascial planes.
Because PCL was diagnosed rapidly and the patient could be started on prompt anthracycline-based chemotherapy with R-CHOP, he was able to tolerate all six cycles without issue, with no evidence of residual disease after the conclusion of therapy. Unfortunately, our patient developed rapidly progressive heart failure from anthracycline toxicity and ultimately was placed on home hospice care.
Conclusion
The prompt recognition of malignant cardiac tumors is a challenge given their overall rarity. The inclusion of CMR should be considered to aid and expedite diagnosis. Prompt initiation of anthracycline-based chemotherapy provides a potential for cure for PCL. Unfortunately, patients are still at risk of cardiotoxicity from anthracyclines, even with remission of their malignancy.