The growing epidemic of cardiovascular disease, together with an ageing population represents a substantial clinical challenge that stretches healthcare budgets. There is an urgent need for both interventions focused on prevention and treatment innovations. In two sessions of presentations at EuroPCR on the 20th May 2014 in Paris, companies presented their latest innovations. The aim of these presentations was to introduce novel technological interventions at the early stages of clinical development.
New Valve and Devices
Permaseal Transapical Access and Closure Device
Professor Willard Hennemann of Micro Interventional Devices Inc., Bethlehem, PA, presented Permaseal™, a device designed to facilitate fast, reliable and reproducible myocardial access and closure in percutaneous transcatheter structural heart repair procedures. The device creates a secure, self-sealing access site into the heart using a unique anchor design, featuring a solid core and flexible valve, which minimises tissue damage. Once the procedure is completed and the cannula and guidewire are extracted, the Permaseal webbing constricts around the opening in the heart, providing instantaneous closure while allowing the flexibility to accommodate a beating heart. The device is available in multiple sizes to accommodate different catheters and sheaths. More than 100 successful preclinical cases have taken place, and the Sutureless Transapical Access and Closure Study (STASIS) is ongoing.
Caval-aortic Access to Allow TAVI in Otherwise Ineligible Patients
Dr Adam Greenbaum of the Institute for Structural Heart Disease, Henry Ford Health System, Detroit, discussed the limitations of current devices enabling transcatheter aortic valve implantation (TAVI) procedures: they require large sheaths, making them unsuitable for large sections of the population; and transapical and transarotic access are associated with vascular complications as well as significant morbidity and mortality. Therefore, an alternative access route is needed. The inferior vena cava (IVC) is close to the aorta without significant intervening structures. Successful caval-aortic access, i.e. percutaneous entry into the abdominal aorta from the femoral vein through the adjoining IVC and closure has been demonstrated in pigs,1 more recently in humans.2 The procedure begins with simultaneous IVC and aortagram. The catheter point is taken to the point of the aorta. The sheath can then be inserted and the TAVI procedure can proceed.
To date, the procedure has been performed 28 times. It is particularly useful in patients with contraindications to transfemoral, transapical and transaortic TAVI, including frailty, inadequate vessel size, prior cardiac, thoracic and aortic surgery. Early outcomes appear comparable to transapical delivery. Successful caval-aortic access has been achieved in 100 % of cases to date, with a mean crossing time of 18 minutes. The procedure is safe, with one mortality and major vascular complications reported in five patients. In conclusion, cavalaortic access allows TAVI in those otherwise ineligible. Furthermore, this procedure may have a role in other transcatheter treatments, such as large devices for aortic insufficiency, thoracic endovascular aortic repair (TEVAR) and left ventricular assist devices.
The LeafLex Catheter System
Professor Sharad Shetty of the Royal Perth Hospital, Australia introduced the Leaflex™ (Pi-Cardia, Beit Oved, Israel) catheter system. In a recent European assessment of TAVI utilisation, 86 % of interviewees thought that the medical needs of treating patients with severe aortic stenosis ineligible for conventional surgery remained unmet. Only 55 % of patients received TAVI: the decision not to refer other patients to TAVI were based on budget, reimbursement, hospital capacity and clinical assessment.3 TAVI is a complex, expensive procedure that entails significant complications and has questionable long-term durability. Balloon aortic valvuloplasty (BAV) is an alternative but was not designed to deal with calcium. Its effect is largely due to short-term stretching of the annulus and restenosis is a problem. The LeafLex aortic valve remodelling therapy (Pi-Cardia) is an innovative option, which fractures calcification in the valve by mechanical impact, resulting in a significant increase in aortic valve area (AVA), and does not involve a permanent implant. The device can be employed as a stand-alone procedure, as a bridge to TAVI or surgical aortic valve replacement (SAVR) or may be used in preparation for TAVI. It achieves effective and controlled impact by using two counteracting elements and produces focal fractures at the leaflet’s original folding points, and therefore breaks the calcification without damage to the leaflets. There is no dilation and no overstretching of the annulus and therefore no risk of annular tear.
Preclinical experience involved more than 200 experiments using a reconstructed aortic valve model. The first-in-human study recruited 15 patients in Europe and Australia and is currently ongoing. The safety endpoints are major adverse cardiovascular and cerebral events (MACCE) at 30 days. Efficacy endpoints include successful device introduction, positioning, operating and withdrawal, and improvement in effective orifice area (EOA) post procedure. In summary, this unique impact procedure has demonstrated good outcomes without generating aortic regurgitation (AR) and without leaflet injury in a reconstructed valve model. The first-in-human study has shown encouraging preliminary results, and will be reported later this year.
Prospective, Bi-centric, Single-arm Pilot Trial to Evaluate Safety and Feasibility of the Transfemoral NVT TAVI System for the Treatment of Severe Aortic Stenosis in High-risk Patients
Dr Peter Wenaweser of the University Hospital, Bern, introduced the Allegra transfemoral TAVI system for the treatment of severe aortic stenosis in high-risk patients. The system comprises a selfexpandable bioprosthesis consisting of a short nitinol stent frame and a bovine pericardial valve. It is implanted transfemorally using a Permaflow deployment system, which avoids outflow obstruction during implantation and enables early functionality of the valve. A prospective single arm pilot trial (n=21) found a 95 % survival rate at 30 days of follow up, and procedural and device success in 91 %; reduction of the mean transvalvular gradient from 49 mmHg to 7 mmHg; and an increase of AVA from 0.6cm2 to 1.7cm2. No AR was reported in 19 % patients, mild AR in 62 %, moderate AR in 9.5 % patients and no severe AR. A New York Heart Association (NYHA) class improvement in was seen in 63 % of patients, no change in 37 % and no deterioration. The results of the pilot trial demonstrate feasibility of the treatment of severe symptomatic aortic stenosis in high-risk surgical patients. In summary, the study demonstrates a high device success rate with a favourable 30-day clinical outcome comparable to commercially available TAVI devices.
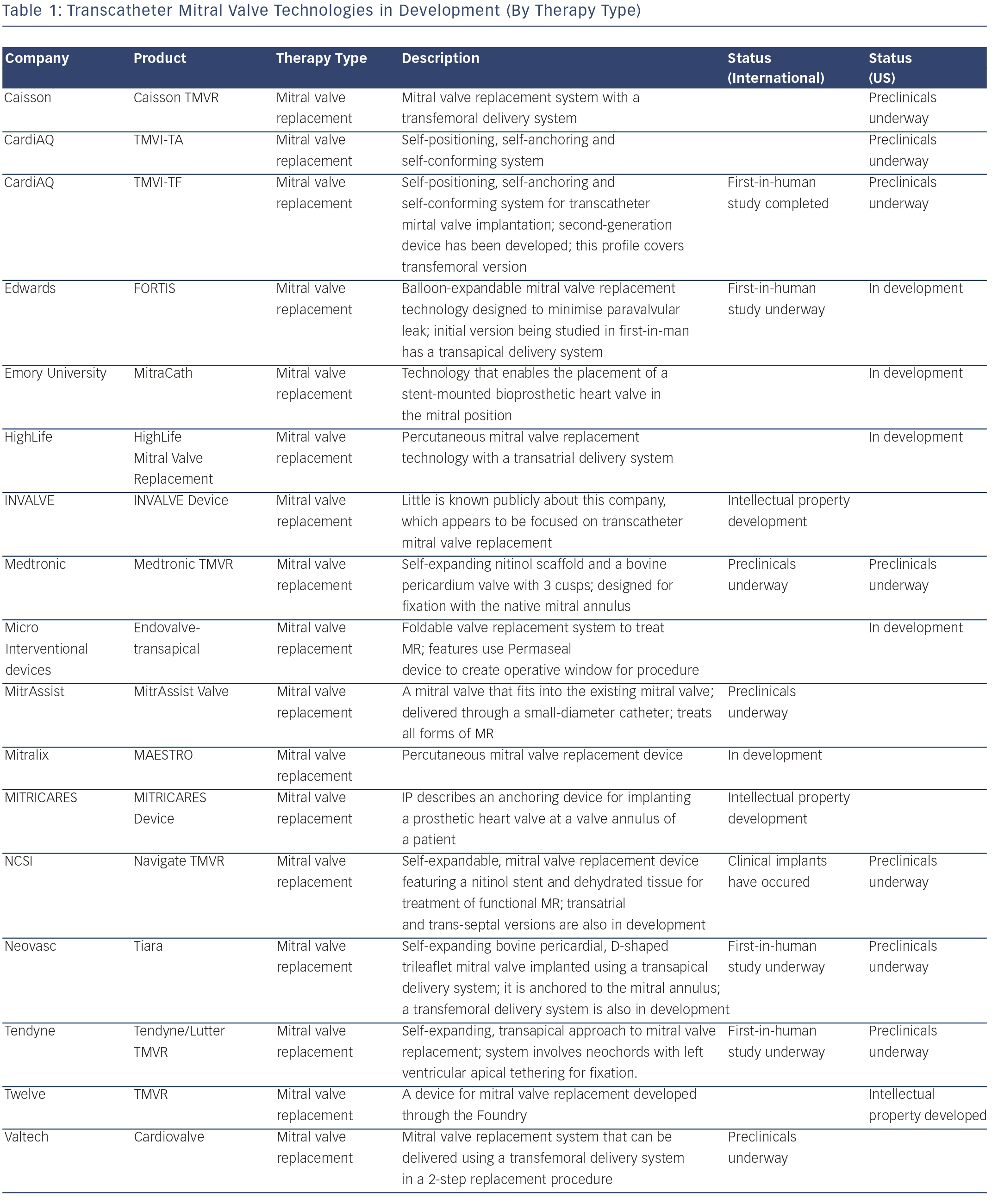
Novel Catheter-guided Mitral Valve Stent for Functional Mitral Regurgitation; Verification in Animal Studies Dr Jose Luis Navia of the Cleveland Clinic, Ohio began by describing two distinct populations of mitral disease: degenerative, in which the mitral valve has a physical deformity due to congenital or rheumatic disease, or functional in which the dilated heart stretches open the mitral valve, causing it to leak or regurgitate. Mitral valve repair or replacement through available surgical platforms is effective in all forms of mitral disease. However, there is a need for percutaneous mitral valve therapy for patients with functional mitral valve regurgitation, especially in high-risk patients unsuitable for open-heart surgery.
The NaviGate transcatheter mitral valve repair (TMVR) system involves a transcatheter mitral valve stent, comprising a temperature shape memory nitinol tapered stent, chemically preserved xenogeneic pericardium, annular winglets for secure anchoring of the annulus and mitral valve leaflet. It has three modes of delivery – transapical, transatrial and trans-septal – all using the same valve configuration. In preclinical studies of acute (24 hours) and sub-chronic (96 hours) implants in swine using fluoroscopy/intracardiac echo for guidance, 12 of 18 animals survived to term, and the implant demonstrated reliable valve performance. In summary, the feasibility of the Navigate TMVR has been demonstrated, and the three delivery options suggest potential ease of implantation.
Percutaneous Remodelling of the Tricuspid Valve with a Novel Cinching Device – Acute and Chronic Experience in an In vivo Animal Model
Dr Francesco Maisano of the University Hospital, Zurich, began by considering the prognostic and economic burden of tricuspid regurgitation (TR). The presence of significant TR is independently associated with a 1.5–2-fold increased risk of heart failure (HF) and death,4,5 and TR is often addressed at the same time as mitral valve interventions. A correlation also exists between annular diameter and TR.6 Multiple techniques for surgical repair exist, including annuloplasty,7 the Kay repair8 and the Hetzer double orifice repair9; however, there is a rising clinical need for a reproducible percutaneous tricuspid valve repair technique as an increasing number of patients are at risk of open-heart surgery.
The TriCinch™ system (4Tech, Galway, Ireland) is a novel transcatheter tricuspid adjustable modelling system. The fixation element is implanted using a steerable delivery system to navigate to the anterior tricuspid annulus. The system is implanted and tensioned under echo guidance to reshape the tricuspid valve and increase the coaption length. A stent is then deployed in the IVC to stabilise the cinching. An in vivo animal study on 31 adult swines (17 acute; 14 chronic, up to 90 days) with annular reshaping, found that the implant was successfully delivered in all cases. The mean procedural time was 43±20.5 min. All animals survived completion of the follow-up. No leaflet or vessel damage, or coronary lesions, were observed, and the implant showed satisfactory healing and integration. The procedure resulted in a significant reduction of valve area, with a 79 % increase in trans-tricuspid peak velocity, as well as a 30 % reduction in septo-lateral dimension and a 70 % increase in tricuspid coaption length. The Percutaneous Treatment of Tricuspid Valve Regurgitation With the TriCinch System™ (PREVENT) first-in-human study, involving 24 patients, trial is ongoing. In conclusion, the TriCinch system has proven feasible and safe in animal models, and initial human studies are under way.
Transcatheter Left Atrial Appendage Closure with Lifetech LAmbre™ Device – First-in-human Experience
Percutaneous left atrial appendage (LAA) closure is increasingly performed in AF patients with high stroke and bleeding risks. However, currently available devices are limited by the need for relatively large delivery sheaths and limited recapture and repositioning capabilities. Dr Yat-Yin Lam of the Chinese University of Hong Kong discussed the LAA occluder – LAmbre™ (Lifetech, Shenzhen, China) – which has an umbrella and a LA cover. It also has a double-membrane design: a distal membrane seals the appendage if that in the cover fails to do so. A titanium nitride (TiN)-coated LA cover with recessed hub promotes faster endothelialisation and reduces delayed thrombus formation. A unique feature is the umbrella, which features eight frames, a polyethylene terephthalate (PET) membrane and eight hooks for multiple capture and repositioning, and has three anchoring mechanisms: hooks, frames and the stenting effect of the oversised umbrella. The device only requires small sheaths (8–10 Fr), and is available in two sizes. The unique delivery system means that distal positioning of the delivery catheter is not required.10
The feasibility and safety of the device has been reported in a canine model.11 Since then, 82 human implants have been performed: 39 as part of the Asian first-in-human registry; 43 in a CE Mark study in Germany. Results of the Asian registry were presented. Procedural time was 65±23 minutes, with successful device implantation on all patients and no significant peri-device leakage. Safety was also acceptable; at 7 days there were two air embolisms and one mild pericardial effusion, all of which was treated conservatively.
In conclusion, preliminary human experience shows that LAA occlusion with the LAmbre device is feasible in various LAA anatomies with no serious peri-procedural events. The main advantages include small delivery system, ease of use and the ability to be fully retrievable and repositionable during implantation.
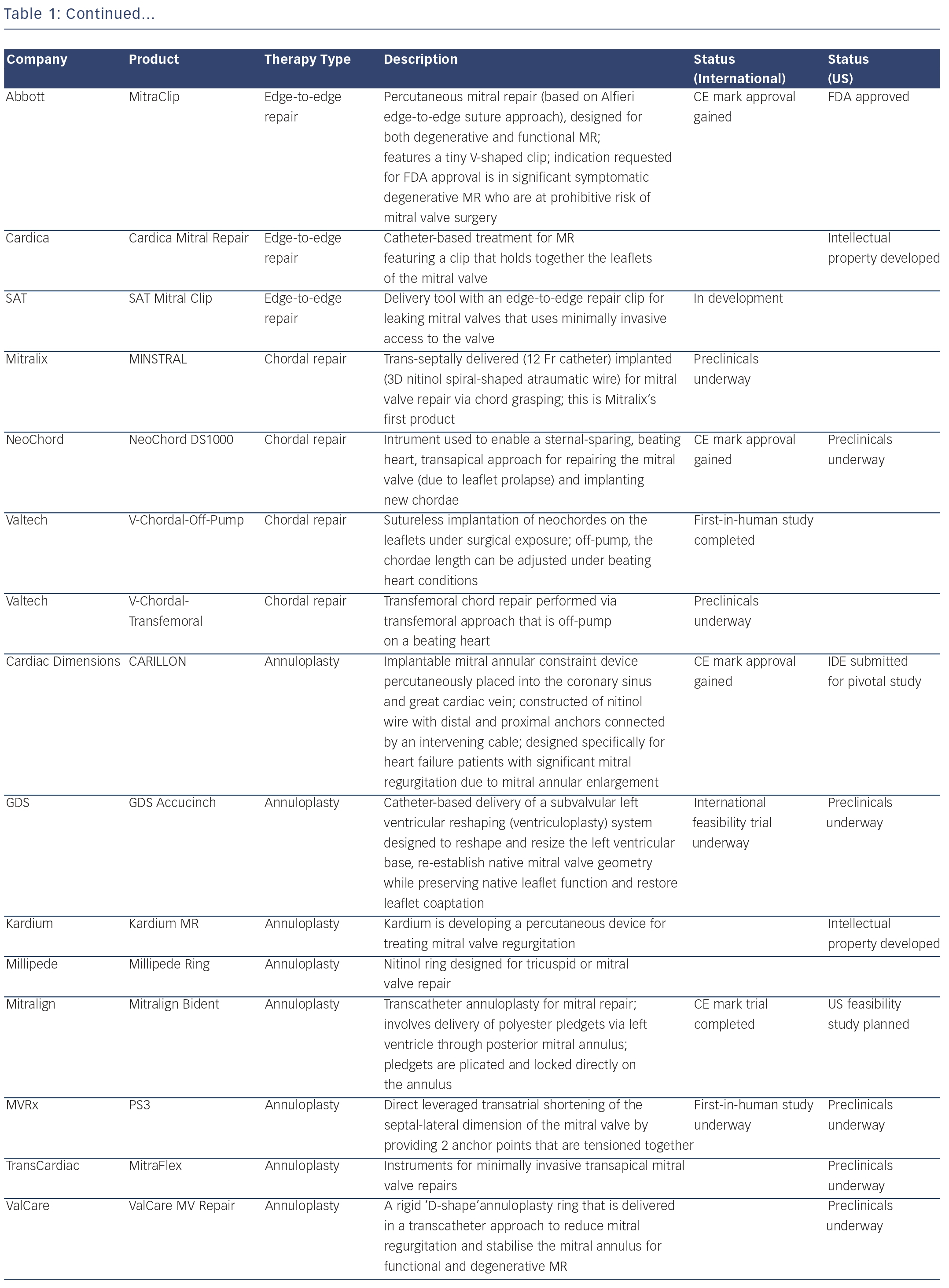
Percutaneous Mitral Ring Annuloplasty – Percutaneous Implantation of Complete Circumferential Annuloplasty Ring
Dr Karl-Heinz Kuck of the Asklepios Clinic, Hamburg introduced the mitral restriction ring cardiac implant, which comprises a multielement circumferential ring with internal cinching wire protected from tissue ingrowth and a pre-set anchor array, delivered with a single shot through a femoral, venous, trans-septal procedure. An implantable actuator enables chronic progressive cinching following completion of tissue healing. The ring is expected to reduce annular dimension and restore leaflet coadaption, restrict further dilatation and provide a clinically significant reduction in mitral regurgitation (MR). Therapy is progressive, relying on natural tissue healing to secure the implant. Progressive cinching allows assessment of MR at 30/60/90 day intervals. Furthermore, if MR occurs, the mitral ring may serve as a retention mechanism for a replacement valve.
Around 25 acute and chronic animal studies in Germany, the US and Israel have been reported. Histological examination revealed that all device components were incorporated within collagenous tissue in-growth except, by design, the internal cinching wire. In conclusion, a complete circumferential ring may become an essential component of TMVR and replacement. The device is easy to use, enabling shorter and less-complex procedures.
New Devices for Coronary Artery Disease and Heart Failure
Liquid Biopsy System – From Design to First-in-human
Dr Nick West of Papworth Hospital, Cambridge, UK began by outlining the rationale behind the liquid biopsy system (LBS). Biomarkers hold the key to determining plaque progression, but current methods of peripheral blood sampling are limited by low concentrations of biomarkers and wide inter-individual variability. This can be explained by the boundary layer phenomenon: plaque-specific biomarkers hug the vessel wall, resulting in inherent sampling difficulties. The LBS employs a catheter with folding mixing structures that disrupt blood flow and break up the boundary layer, diverting biomarkers to the centre of the vessel. Simultaneous sampling at four points downstream of a plaque will therefore contain enhanced concentrations of biomarkers released by the plaque.
Preclinical data in a porcine model confirmed the device safety. The first-in-human study is a single-arm observational cohort study (n=30) to demonstrate the safety of LBS as an adjunct to percutaneous coronary intervention (PCI). The procedure met all performance and safety endpoints with 100 % procedural success and no significant AEs. In conclusion, use of the LBS for intracoronary blood sampling may enable better identification of plaque-specific biomarkers and identify plaque gradients, improving understanding of plaque progression, as well as potentially providing novel therapeutic targets and surrogate endpoints for clinical studies.
Intra-aterial Applications of Biodegradable Nanoparticles Loaded with Everolimus.
Professor Krzysztof Milewski of the American Heart of Poland SA project presented the concept of biodegradable nanoparticles. Nanoparticles must measure between 1 and 100 nm in at least one dimension. This structure confers unique properties including enhanced reactive area and the ability to cross cell and tissue barriers. This should theoretically allow substantially increased intracellular drug intake, increased in vivo drug stability and prolonged and controlled drug release, and will be attractive in applications where stents are not suitable or when multiple drug injections or combinations are required. The benefits of intra-arterial drug delivery systems based on paclitaxel have been demonstrated.12
Intra-aterial applications of biodegradable nanoparticles loaded with everolimus is achieved using a microporous delivery catheter: Clearway® (Atrium Medical, Hudson, NH). In a phase II study involving 22 porcine coronary segments, local delivery of 100 μg of everolimus encapsulated in nanoparticles dissolved in 2 ml of normal saline was shown to be feasible, achieving high drug concentrations in tissue and a steady decrease over 90 days. In an ongoing phase III study of 24 porcine coronary segments, optical coherence tomography (OCT) and histopathological analysis were performed at 28 and 90 days, as well as immunofluorescence technique to define nanosphere distribution at early timepoints. Neointimal area at both timepoints was comparable to that seen following implantation of a bare metal stent (BMS), In summary, nanoparticle use will aim to improve the safety and, if possible, the efficacy of interventional procedures. Detailed histopathological analysis will help to determine the reason for a discrepancy between high drug tissue and lack of efficacy found in OCT.
First-in-human Study of XINSORB Scaffold for Patients with Single de novo Coronary Lesions
Dr Hunbo Ge of Zhongshan Hospital, China, introduced the XINSORB™ (Shanghai Weite Biotech, CO.; Ltd., China) resorbable polymer scaffold with an expandable stent carrying sirolimus. The first-in-human study (n=30, A/B1 type single de novo coronary lesions) is ongoing, and involves clinical assessment at 1, 3, 6, 9 and 12 months and angiographic follow-up at six months. Following a successful preclinical study,13 preliminary human data have shown that the success of the procedure was 100 %, and no major adverse cardiac events (MACE) occurred at one and three months follow-up. Six-month follow up data of the first patient treated with the XINSORB scaffold show that almost all struts were covered by neointima (thickness 100 μm) and there was no stent malapposition. Excellent intimal healing was reported without apparent scaffold structure remodelling. The long-term effect of the XINSORB scaffold in human coronary arteries therefore deserves further large-scale investigation.
The ENABLER System – Chronic Total Occlusion Crossing Device
Dr Maurice Buchbinder of Stanford University, CA introduced the ENABLER crossing catheter system (EndoCross Ltd, Yokneam Illit, Israel), a novel means of entering an area of occlusion. An ENABLER catheter advances over the guidewire towards the lesion, a lowpressure balloon inflation within the catheter anchors the system against the vessel wall proximal to the occlusion and a cyclic pressure control unit is activated, creating repetitive balloon elongation and contraction that results in guidewire gripping and progressive advancement at 1 mm per cycle. The ENABLER is the only crossing device that automatically adjusts the wire position, thus helping to maintain luminal positioning.
The first-in-man study (n=37, including heavily calcified, long and fibrotic occlusions) demonstrated successful crossing in 86 % of cases. The average activation time for successful crossing was 5.3 minutes (range 0.4–22). Only one (3 %) device-related complication occurred, a perforation when the wire was advances into a side branch, which was managed conservatively without further sequelae.14 In conclusion, the ENABLER system, which provides enhanced force to a standard guidewire tip for controlled intraluminal advancement, is a promising device for the treatment of peripheral chronic total occlusions (CTO).
Intra-aortic Balloon Pump – Novel Insights into Mechanism of Action
Dr Nico Pils of Catharina Hospital, Eindhoven, the Netherlands, began by presenting a clinical enigma. In some patients with a large ST-elevation myocardial infarction (STEMI), there is a dramatic improvement in clinical condition following use of an intra-aortic balloon pump (IABP); in others no effect at all. The mechanism of action of IABP is an increase of diastolic aortic pressure that increases coronary blood flow and myocardial oxygenation, relieving ischaemia and improving cardiac output. However, it is also necessary to consider coronary autoregulation, whereby a change in diastolic blood pressure causes compensatory dilation of contraction of arteriolar sphincters. As a result, coronary blood flow remains equal over a wide range of perfusion pressure.
Under normal physiological circumstances, an increase of diastolic perfusion pressure does not increase coronary blood flow and oxygen supply; autoregulation counters the IABP effects. Only in cases of exhausted autoregulation can an increase of coronary blood flow be explained from augmented diastolic aortic pressure, i.e. in a large STEMI with ongoing ischaemia. In other words, IABP is expected to be useful in patients with viable myocardium, suffering from persistent ischaemia despite an unobstructed coronary artery. This is often seen after successful epicardial stenting following a large STEMI and is clinically reflected by persisting ST elevations and ongoing chest pain. This hypothesis has been validated in an ex vivo beating heart porcine model.
Transfemoral PulseCath® Left Ventricle Assist Device
Dr Robert-Jan van Geuns of Erasmus MedicalCenter, Rotterdam, the Netherlands introduced the transfemoral PulseCath® (PulseCath BY, Amsterdam, Netherlands) left ventricle (LV) assist device, a transaortic pump that fills the gap between minimally invasive IABP and the complex, expensive implantable or external ventricular assist devices. The PulseCath is driven by a normal IABP console, and actively unloads the LV during systole and expels blood to the descending aorta during diastole. Its main components are a single lumen catheter, a membrane pump that generates a blood flow of up to 2.2. l/minute and a two-way valve.
The first-in-human study in 2013 (n=15) demonstrated successful introduction of the device without complications in 100 % of patients with an output of 1.5 l/minute. Limitations observed were the need for a large access site and the fact that cardiac output was dependent on heartrate. The PulseCath received the CE mark for up to 24 hours’ use in February 2014, and larger clinical studies are planned in Europe. A new device enabling a 3 l/minute pulsatile flow is also in development.
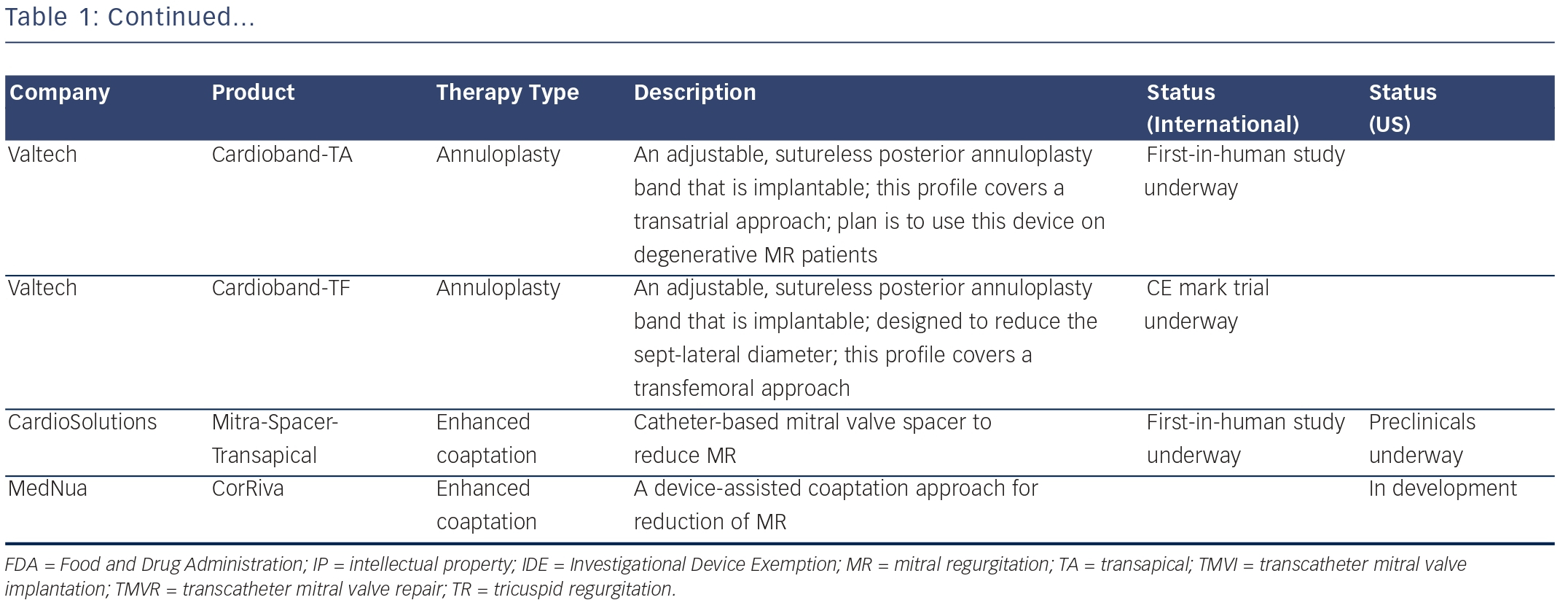
Left Atrial Decompression through Unidirectional Left-to-right Shunt for the Treatment of Left Cardiac Failure – Initial Experience with the V-wave Device
Dr Josep Rodés-Cabau of the Quebec Heart and Lung Institute, Canada, began by emphasising the importance of continuous progress and innovation in improving the treatment of HF. Patient-directed selfmanagement improves outcomes in advanced HF,15 and there are data to support the use of left heart decompression.16–18 The V-wave device is a permanent implant for heart failure patients with elevated left atrial filling pressure, which creates a left to right shunt at the atrial septum and is introduced by trans-septal puncture at the LV.
A study in ovine models of chronic HF (n=14) demonstrated successful implantation in all cases, without any adverse events (AEs).19 The firstin- man study enrolled five patients with chronic left HF. Successful implantation was reported in all cases, and all patients were discharged at 24 hours with no complications. The procedural time was 58 (43–65) minutes. Permeability of the device was seen immediately and at 24 hours in all patients. Significant improvements in functional status, quality and life and haemodynamic parameters were reported at 90 days. In summary, the successful and uneventful first-in-man experience with the V-wave device showed the feasibility of applying this new therapy in HF patients, and warrants further study.
Hybrid Transcatheter Therapy for Ischaemic Cardiomyopathy Heart Failure
Dr Lon Annest of BioVentrix, USA described a new surgical procedure, the Revivent-TC™ Ventricular Enhancement System, used to reshape and reduce the LV. In this procedure, small titanium anchors are placed along the outer surface of the heart and along one of the interior walls via a catheter-based approach. The anchors are then pulled towards one another, effectively excluding the scarred and non-functioning heart wall. This hybrid procedure involves a cardiologist and a surgeon. The Revivent-TC System utilises a myocardial anchor identical to the Revivent™ Myocardial Anchoring System, which received a CE mark in December 2012, and the efficacy of which has been demonstrated in clinical studies.20 To date, eight hybrid cases have successfully been completed Revivent-TC Ventricular Enhancement System.
Summary and Concluding Remarks
Open heart surgery remains a procedure that many patients are reluctant to undergo. New techniques with minimal access and percutaneous interventions are increasingly being introduced to catheter labs, angiography suites and operating theatres. New technologies, allowing novel modes of access, valve remodelling and new treatment options for HF have shown promise in early clinical studies. In summary, technology in the field of mitral valve disease, coronary artery disease and HF continues to advance to meet patient demands.