Anatomy and Classification
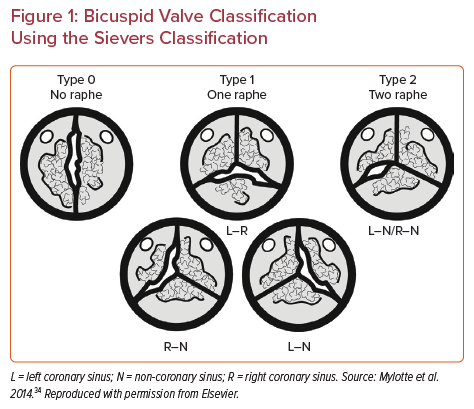
Bicuspid aortic valve (BAV) is the most common congenital cardiac abnormality, affecting around 2% of the population. BAVs are structurally abnormal valves with different morphologic phenotypes. Sievers and Schmidtke classified BAV into three types based on the number of fused raphes: type 0 with no raphe, type 1 with one raphe and type 2 with two raphes (Figure 1).1 The three types are then divided into subcategories based on the spatial position of the cusps (type 0) or the raphe (types 1 and 2).1
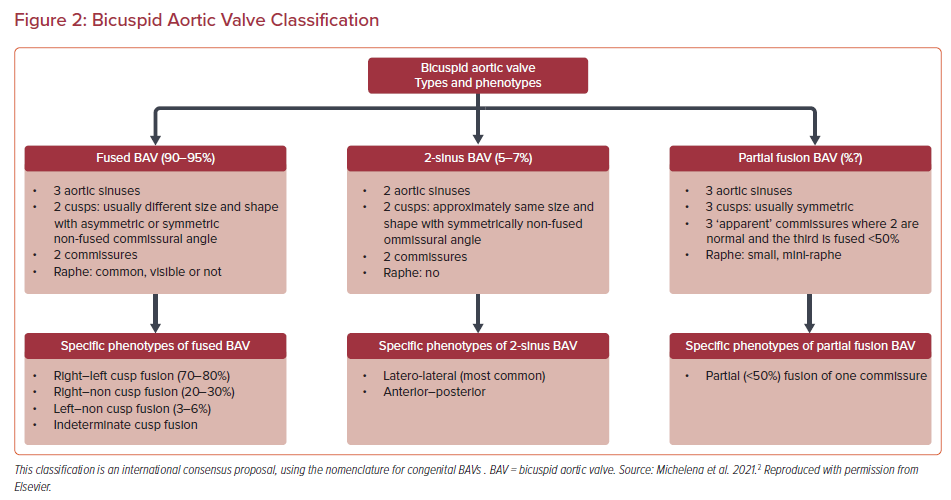
The morphologic classification of BAV is expanding and new systems have been proposed, including a recent consensus statement from major societies to account for additional BAV phenotypes. The new proposed system is divided into fused BAV, 2-sinus BAV and partial fusion BAV.2 Fused BAV has three sinuses, two cusps and two commissures, 2-sinus BAV has two sinuses and two cusps, and partial fusion BAV has three sinuses, three cusps and three commissures but one is <50% fused (Figure 2). BAVs are associated with an increased risk of valvular disease as well as aortic dilatation and complications. The pathophysiology of BAV aortopathy is complex, with proposed interactions between both underlying aortopathy and abnormal flow dynamics through the BAV.3 The genetic basis of BAV has not been fully characterized but studies have identified candidate genes and demonstrated autosomal dominant inheritance with reduced penetrance and variable expressivity.4
Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve
BAVs are associated with an increased risk of both stenotic and regurgitant valvular disease. The management of aortic stenosis has been transformed in recent years due to the introduction of transcatheter aortic valve replacement (TAVR). TAVR has been to shown to be a safe and effective means of therapy for aortic stenosis with approval in all patient risk categories; however, the majority of the data and clinical trials have been focused on tricuspid aortic valves. The management of the stenotic BAV poses additional challenges in anatomy and clinical decision-making. Current guidelines do not make firm recommendations for the management of the stenotic BAV in regard to surgical aortic valve replacement (SAVR) versus TAVR. There are many considerations in BAV patients that a heart team must consider when managing these challenging cases.
Surgical Aortic Valve Replacement in Bicuspid Aortic Valve
As TAVR has matured over the last decade and continues to improve, SAVR has evolved as well with multiple advancements in the armamentarium for surgeons. These include advancements in mechanical heart valves with approved lower international normalized ratio targets in sutureless and rapid deployment (SURD) valves, and the Ross procedure.5 The advantage for SURD is the ease and speed of implantation, which can aid in facilitating minimally invasive and multivalve procedures. The Ross procedure involves explanting the patient’s pulmonary root and valve as an autograft and implanting it in the aortic position with a pulmonary homograft to replace the explanted pulmonic valve. The advantage of the Ross procedure is extended durability without the need for long-term anticoagulation. There are also data to suggest that the patient’s life expectancy is restored to that of a matched group without aortic stenosis.6
The use of SURD in BAV has been traditionally considered as contraindicated. However, this has been challenged, with multiple groups presenting data showing excellent results in BAV including Sievers type 0 valves. Nguyen et al. first published a series of 25 BAV patients who had an SAVR with SURD.7 They had no cases of valve embolization and only one valve required redeployment. The rate of mortality was low at 4%, with a new permanent pacemaker (PPM) rate of 20%. However, it is important to note that none of the patients in that series had Sievers type 0 BAV, which has been considered particularly unfavorable for SURD. Vola et al. reviewed the early experience with the 3f Enable sutureless prosthesis (Medtronic) and identified five patients out of 200 patients who received the 3f Enable who had Sievers type 0 morphology.8 Two patients out of five had 1+ paravalvular leak (PVL) intraoperatively and one had the PVL worsen to 2+ during follow-up. The authors raised the concern that the elliptical nature associated with type 0 BAV in particular may be associated with a higher risk of PVL and caution should be taken if using SURD. Durdu et al. published a report on a series of elderly patients with BAV undergoing SURD SAVR through a minimally invasive right anterior thoracotomy approach.9 They included four patients with type 0 BAV out of a total of 13 patients. They were able to achieve excellent results with only one patient having 1+ or greater PVL with zero mortality. The international experience with SURD was reviewed in the SURD international registry, in which 191 patients out of 4,636 had BAV.10 The outcomes were excellent, with a high usage of minimally invasive approach (73.8%) and low mortality (1.6%). The rate of moderate or greater PVL was 3.9%. However, the type of Sievers morphology was unavailable in the registry. It is probable that the majority of these cases were Sievers type 1 valves but the registry suggests that SURD can be safely used in selected BAV patients.
The Ross procedure has waxed and waned in popularity, with a revival in recent years prompted by advancements in surgical technique. The use of the Ross procedure in BAV has multiple advantages in the younger patient population, with the avoidance of long-term anticoagulation, favorable hemodynamics and extended valve durability. However, the use of the Ross procedure in BAV has been controversial, with concerns about long-term durability with autograft dilatation. This was reviewed in a large population of 1,277 patients from the German Ross registry with a mean follow-up of 5.7 years.11 In the registry 70.9% had a BAV and there were no differences between BAV and tricuspid valves in the development of aortic insufficiency. However, the BAV patients did have a higher rate of annular dilatation (0.20 versus 0.06 mm/year, p=0.003) and sinus dilatation (0.24 versus 0.11 mm/year, p=0.013). Although there was no difference in aortic insufficiency the 5.7-year follow-up is short when looking at this young patient population (mean age, 42.2 years), and the difference in annular and sinus dilatation raises concerns for long-term durability.
The effect of types of cusp fusion in BAV on autograft dilatation and insufficiency was studied by Ruzmetov et al. in 43 pediatric patients over a 9.6-year follow-up.12 They found that the left–right fusion phenotype versus the right-non phenotype was associated with higher rates of autograft valvular insufficiency > moderate (39% versus 6%, p=0.03). This raises concern for the use of the Ross procedure in certain types of BAV morphology. The use of the Ross procedure in pure aortic insufficiency has also raised controversy, and the presence of BAV raises further concerns. This was examined by Poh et al. in 129 consecutive patients with BAV and pure aortic regurgitation.13 They noted excellent results, with a 20-year freedom from re-do aortic valve replacement and greater-than-mild aortic regurgitation of 85%. Our understanding of the Ross procedure is still increasing, and in selected centers of excellence it can be an excellent option in the treatment of aortic valve disease in younger patients.
Transcatheter versus Surgical Aortic Valve Replacement
Patients with BAV have a higher risk of developing aortic stenosis and do it at a younger age than patients with tricuspid valves. This accounts for up to 50% of SAVRs in younger patients.14 The age of the patient is an important consideration in the decision-making of SAVR versus TAVR because the effect of durability and any complications such as PVLs and pacemakers may be amplified. TAVR is a newer technology with less long-term durability data as compared with SAVR. Reintervention after TAVR was higher at 5 years than after SAVR in the PARTNER-II trial (3.2% versus 0.8%).15 However, the rate of structural valve deterioration in a propensity-matched registry was not different between TAVR and SAVR when looking at the newer generation balloon-expandable prosthesis, suggesting that the durability may be similar between newer generation TAVR and SAVR.16 Long-term durability data have been reported only to 10 years from the initial TAVR trials. The data appear promising but it is difficult to ascertain durability due to competing risks related to the high-risk profile of the initial TAVR patients.17
Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve: Potential Complications
Randomized controlled trials have shown a trend towards a higher PVL rate with TAVR versus SAVR in tricuspid aortic valves.15 This is also seen in BAV patients who have had TAVR (2.7% versus 2.1%).18 This is concerning because patients with moderate or greater PVL have been shown to have a higher mortality rate, but the impact of mild PVL is unclear.15 The 5-year results from the PARTNER II trial showed a 33% rate of mild PVL with TAVR as compared with 6.3% in the SAVR group.15 This high rate of mild PVL was in the tricuspid population and may be even higher in a patient with BAV. However, the PARTNER II trial used an earlier generation of balloon-expandable valves, and the PARTNER III trial noted less PVL but BAV patients were excluded.6 The clinical significance of mild PVL is unclear but its impact may be increased in a younger patient who will have PVL for a longer period of time. An analysis of 17 studies and 181,433 patients found the risk for moderate to severe PVL to be higher in subjects with BAV than in those with tricuspid aortic valve (RR 1.42; 95% CI [1.29–1.58]; p<0.0001).19
PPM implantation following TAVR has been associated with increased mortality.20 Randomized controlled trials looking at self-expanding TAVR valves versus SAVR have noted a higher rate of PPM with TAVR (17.4% versus 6.1%).21 However, this finding was not seen with balloon-expandable valves, with a PPM rate of 2.4% with TAVR and 2.9% with SAVR.22 It is thought that TAVR in BAV would have higher rates of PPM due to multiple anatomic reasons such as higher calcium burden and asymmetric annuli. In the Society of Thoracic Surgeons (STS) and Transcatheter Valve Therapies (TVT) Registry there was a slightly higher rate of PPM implant with BAV with a hazard ratio of 1.23 (7.5% versus 9.1, p=0.05).23 Interestingly, despite the indication that self-expanding valves have a higher rate of PPM than balloon-expandable valves in tricuspid aortic valves, this may not be true in BAV. In the BEAT (Balloon versus Self-expandable Valve for the Treatment of Bicuspid Aortic Valve Stenosis) Registry there was no difference between self-expanding and balloon-expandable valves in the PPM rate (16.0% versus 16.1%, p=0.977).24 There are limited data to evaluate the association of BAV morphology and need for PPM but Yoon et al., using the International Bicuspid Aortic Stenosis Registry, saw a trend toward a higher PPM rate in BAV with more calcified raphe or leaflets, although this was not statistically significant (p=0.23).25
Earlier studies have demonstrated higher risks of stroke in bicuspid versus tricuspid aortic stenosis. A systematic review and meta-analysis of 17 studies noted that the incidence of cerebral ischemic events was higher for bicuspid versus tricuspid aortic stenosis (2.4% versus 1.6%; p=0.015).19 The 30-day stroke rate reported by Makkar et al. was significantly higher for bicuspid versus tricuspid aortic stenosis (2.5% versus 1.6%).23 The TORCH (Transcatheter Aortic Valve Replacement in Chinese population) study assessed the risk of brain injury in patients with BAV following TAVR.26 All patients received diffusion-weighted brain MRI before and within 6 days after TAVR. The findings suggested a higher number and size of cerebral ischemic lesions. That study found that the overt stroke rate was 2.4% in BAV patients and 1.7% in tricuspid aortic valve patients, which were comparable to findings by Makkar et al.15 More recent data from the STS/TVT Registry compared the outcomes of patients with BAV with those of patients with tricuspid valves undergoing TAVR with the self-expanding valve.27 Stroke rates were similar for the two groups at 30 days (3.4% for bicuspid patients versus 2.7% for tricuspid patients) and at 1 year (3.9% for bicuspid patients versus 4.4% for tricuspid patients). Most bicuspid patients were at intermediate or high surgical risk. Given earlier data demonstrating increasing stroke risk, some institutions have incorporated the use of embolic protection devices.23 There are, however, currently no randomized data to support this practice.
Sizing, Valve Choice and Bicuspid Aortic Valve Morphology
The elliptical nature of the stenotic BAV with its larger annular dimension may impair appropriate placement of the transcatheter aortic valve. This, combined with more calcified and asymmetrical leaflets, may limit appropriate and circular expansion of the prosthetic valve. Two different methods of sizing have been suggested, annular and supra-annular sizing. The recommended method for supra-annular sizing is to measure the inter-commissural distance 4 mm above the annular plane. Supra-annular sizing is less reproducible than annular sizing, with no difference in procedural complication rates.28 Most programs have reverted back to annular sizing.
A meta-analysis of observational studies of transcatheter aortic valve implantation (TAVI) for bicuspid valves showed no difference in short- and mid-term TAVI mortality with balloon-expandable versus self-expanding valves.19 The balloon-expandable valves had a statistically significantly higher risk of annulus rupture (2.5%) compared with self-expanding valves (0%) (OR 5.81; 95% CI [3.78–8.92]; p<0.001).19 New-generation balloon-expandable valves were also associated with significantly less PVL than new-generation self-expanding valves (OR 0.08; 95% CI [0.02–0.35]; p=0.001).19 The BEAT Registry compared 353 patients who underwent TAVR with the new-generation Evolut R/PRO or Sapien 3 valves in BAV.29 VARC-2 device success was obtained in 86.7% of patients, without significant differences after self-expanding valve implantation and balloon-expanding valve implantation for both the entire population and the propensity score-matched cohort. The rate of moderate–severe PVL after TAVI was higher after self-expanding valve implantation at 1 year in the propensity score-matched population. Self-expanding valves had better hemodynamics at discharge and 1-year follow-up with lower mean gradient and higher effective orifice area. At 30-day follow-up and at 1-year follow-up the two treatment groups had similar rates of clinical events both in the entire cohort and in the matched population despite the hemodynamic difference.
With current-generation TAVR devices (Sapien 3 and Evolut R), the incidence of device success (96.3% versus 97.4%; p<0.001) was only slightly lower for patients with BAV versus tricuspid aortic valve; and the residual 2+ aortic insufficiency (2.7% versus 2.1%; p=0.006) remained slightly higher for patients with BAV versus tricuspid aortic valves. The use of current-generation balloon-expandable valves was associated with a lower risk of significant PVL in patients with BAV than current-generation self-expanding valves.18
BAV morphology has been shown to have a significant effect on the clinical outcomes of TAVR. This is related to both the Sievers type morphology as well as the level of raphe and leaflet calcification. Yoon et al. reported that calcified raphe and excessive leaflet calcification were found to be risk factors for mortality in patients with BAV undergoing TAVR.25 They also found that Sievers type 0 was not associated with worse clinical outcomes. The BEAT Registry reported similar results in that there was no difference in clinical endpoints, but Sievers type 0 BAV was associated with a higher incidence of elevated mean gradient >20 mmHg and a trend towards lower VARC-2 success rates.20 Further anatomical considerations in TAVR for BAV include the distribution and presence of significant calcification. BAV tends to be associated with higher calcium burden.30 Left ventricular outflow tract calcium in particular is associated with higher rates of root rupture.31 The distribution and presence of calcium in BAV, along with Sievers classification, should also be considered in the decision-making process.
Bicuspid Aortic Valve Aortopathy
BAV is associated with a higher risk of aortic aneurysms and aortic complications such as dissection or rupture. Recent guidelines from the American Heart Association have addressed the management of aortic dilatation or aortopathy with BAV. According to the 2020 American College of Cardiology/American Heart Association guidelines for the management of patients with valvular heart disease, the indications for intervention on a dilated aorta in a patient with BAV are similar to those for trileaflet aortic valves.32 The class 1 recommendation is to replace the ascending aorta or aortic root if the size is greater than 5.5 cm. However, for BAV patients there is an additional class 2a recommendation for intervention at a diameter of 5.0 cm if there are risk factors for dissection and the surgery is performed at a comprehensive valve center. For patients undergoing SAVR, the recommendation is to replace the ascending aorta or aortic root if the diameter is >4.5 cm for both BAVs and tricuspid valves. The evidence and recommendations, however, are unclear in the era of TAVR. If there is an intermediate or high-risk patient with a dilated ascending aorta of 4.5 cm and aortic stenosis, should that sway the needle towards SAVR? Other factors that should make SAVR favorable over TAVR would be a need for concurrent left internal mammary artery for coronary disease, unfavorable distribution of calcium in the left ventricular outflow tract, age <70 years and, specifically if a mechanical valve is desired, multivalvular disease, type 0 morphology, severe eccentricity of the annulus and a horizontal aorta, especially in the case of self-expanding valves that may have challenges with co-axiality. Given the significant complexity and planning involved in the treatment of BAV it is imperative to perform such procedures at experienced centers with higher operator volumes. Availability of a robust surgical program that is proficient in first-time SAVR with concomitant aortic root enlargement, may pave the way for better hemodynamic profiles should a need for TAVR arise in the future. Conversely, an experienced surgical program is needed for high-risk aortic surgery such as TAVR explant with or without aortic and/or mitral surgery should a transcatheter valve fail.
Conclusion
Decision-making in the management of patients with stenotic BAV is difficult and highlights the utility and strength of the heart team approach. BAVs have a wide spectrum of morphology that can be accompanied with varying degrees of aortopathy. The patients are clinically diverse and range from low risk and young to comorbid and elderly. Our understanding of the role of TAVR in BAV is evolving with ongoing clinical trials and our understanding of BAV and accompanying aortopathy is expanding as well. When considering the lifetime management of patients with aortic valve disease, both TAVR and SAVR need to be discussed by the heart team. The EXPLANT TAVR Registry showed that a high percentage of patients (34%) were ineligible for repeat TAVR.33 SAVR following TAVR comes at a significant mortality burden (in-hospital, 30-day, and 1-year mortality rates were 11.9%, 13.1%, and 28.5%, respectively; stroke rates were 5.9%, 8.6%, and 18.7%, respectively) compared with first-time SAVR.33 These high rates could be explained by the concomitant need for mitral valve surgery (32%) and aortic root surgery (17.9%) during TAVR explantation, which may have contributed to these numbers. Up to 25% of patients from the EXPLANT TAVR Registry were low-risk candidates; although a breakdown of how many of these were bicuspid is not provided, there is a compelling case to offer SAVR as a first-line option in cases of BAV unless it is of prohibitive risk or due to patient preference. Heart teams must recognize these data and inform patients about the risks associated with the need for TAVR explantation during the decision-making process. Optimal management of these patients requires careful thought and an understanding that no two valves are the same and no two patients are the same.