Coronary artery disease (CAD), the most common cause of morbidity and mortality in the US, is frequently identified by coronary angiography. Decisions for treatment are often based on angiography alone, absent other clinical indicators for intervention. However, by angiography alone, conventional wisdom has suggested that a coronary stenosis is significant if there is at least a 50 % diameter reduction in the left main coronary artery, or at least a 70 % diameter reduction in any other epicardial artery. Over the past two decades, the use of in-lab coronary physiology has demonstrated that angiography alone is not accurate in determining ischemia for intermediate lesions. Compared with angiography aloneguided percutaneous coronary intervention (PCI), physiology-guided PCI is associated with improved clinical outcomes and cost-effectiveness. The most commonly used invasive physiologic indices include fractional flow reserve (FFR), coronary flow reserve (CFR), and instantaneous wave-free ratio (iFR). A solid understanding of coronary physiology and the tools for measuring it is paramount to good clinical decision-making for anyone practicing invasive and interventional cardiology. With this in mind, the purpose of this review will be to identify and explore some of the most recent developments and controversies in invasive coronary physiology.
Fractional Flow Reserve Foundations
It is now well known that FFR is the ratio of the mean coronary pressure distal (Pd) to a stenosis to the mean aortic pressure (Pa) at maximal hyperemia or when myocardial resistance is at its presumed absolute minimum. This condition permits pressure and flow to be linearly related, and thus the ratio represents the fraction of normal coronary blood flow across a stenosis with a normal value being 1 (i.e. Pd=Pa). FFR requires the induction of maximal hyperemia, most commonly with an intravenous infusion or intracoronary bolus of adenosine. Studies have shown that FFR is comparable to noninvasive functional testing. Originally an FFR <0.75 was considered significant and indicates a reduction in coronary pressure by 25 % of normal. To improve the sensitivity of FFR the threshold has been raised to 0.80 and it is the threshold endorsed by the American College of Cardiology (ACC), American Heart Association (AHA), Society for Cardiovascular Angiography and Interventions (SCAI), and European Society of Cardiology (ESC) in their guidelines for PCI.
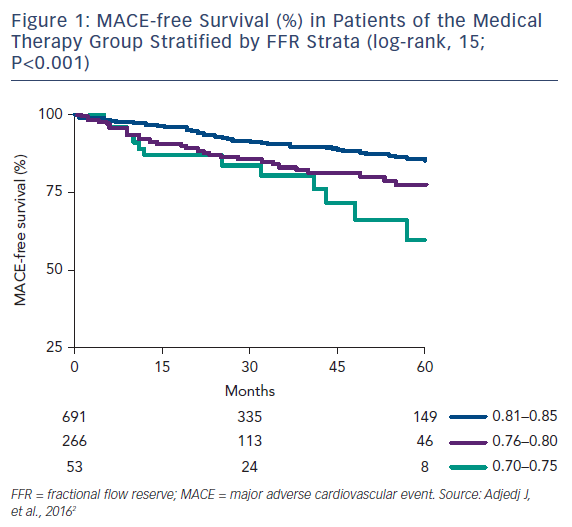
This threshold has left a clinical decision-making “gray zone” FFR of 0.75–0.80. A recent study by Agarwal et al. found that among 238 patients with gray-zone FFR, revascularization was associated with a significantly reduced risk of major adverse cardiovascular events (MACE) compared with medical therapy alone (Figure 1).1,2 On the other hand, in the Interventional Cardiology Research Incooperation Society Fractional Flow Reserve (IRIS-FFR), a large prospective, multicenter registry, the risk of MACE was not significantly different between deferred and revascularized lesions for FFR ≥0.76 (including the gray zone).3 In these situations, the decision to revascularize based on the clinical context of the patient is still best left to the clinician.
Fractional Flow Reserve for STEMI Revascularization Guidance
The utility of FFR to guide revascularization of intermediate lesions in patients with chronic stable multivessel coronary artery disease has been validated in several landmark clinical trials including the Fractional Flow Reserve to Determine the Appropriateness of Angioplasty in Moderate Coronary Stenosis (DEFER), FFR versus Angiography for Multivessel Evaluation (FAME 1), and FFR-guided Percutaneous Coronary Intervention Plus Medical Treatment Versus Medical Treatment Alone in Patients With Stable Coronary Artery Disease (FAME 2).4–6 The utility of FFR in acute coronary syndrome (ACS) has been controversial. During a ST-segment elevation myocardial infarction (STEMI), the degree of damage to the infarct-related or culprit myocardial bed makes FFR in this artery questionable. Furthermore, whether or not the non-culprit myocardial bed is impaired limiting the usefulness of FFR is unknown. Changes in microcirculation and coronary flow secondary to both the plaque activation and myocardial tissue infarction make the feasibility and accuracy of measuring FFR during STEMI (i.e. the ability to induce maximum hyperemia) unreliable. A subhyperemic response to adenosine would result in an underestimation of stenosis severity so that an FFR >0.80 at the time of STEMI may initially be considered nonsignificant, but it may decrease several days later as the injured myocardial bed recovers and flow increases to the area.
Traditional teaching has been to treat only the culprit vessel during STEMI because several meta-analyses and non-randomized registry studies showed that treating all vessels at the same time as the STEMI culprit was associated with more adverse events. Up until the most recent 2015 update, the 2013 ACC/AHA/SCAI STEMI guidelines gave a class III recommendation against intervening on a noninfarct-related artery at the time of primary PCI in patients who are hemodynamically stable.7 Since then several studies have shown the accuracy of FFR in ACS, including Fractional Flow Reserve Versus Angiography in Guiding Management to Optimize Outcomes in Non-ST-Elevation Myocardial Infarction Cardiac Magnetic Resonance (FAMOUS NSTEMI CMR) substudy that showed an excellent accuracy of FFR <0.80 for predicting perfusion defects on cardiac magnetic resonance.8 Furthermore, multiple trials including Preventive Angioplasty in Acute Myocardial Infarction (PRAMI), Complete Versus Lesion-Only Primary PCI Trial (CvLPRIT), and the Third Danish Study of Optimal Acute Treatment of Patients With STEMI: Primary PCI in Multivessel Disease (DANAMI3– PRIMULTI) have shown that revascularizing non-culprit arteries, either at the time of primary PCI or later in a staged manner, reduce risk of MACE by a relative 44–65 % compared with culprit-only PCI.9–11
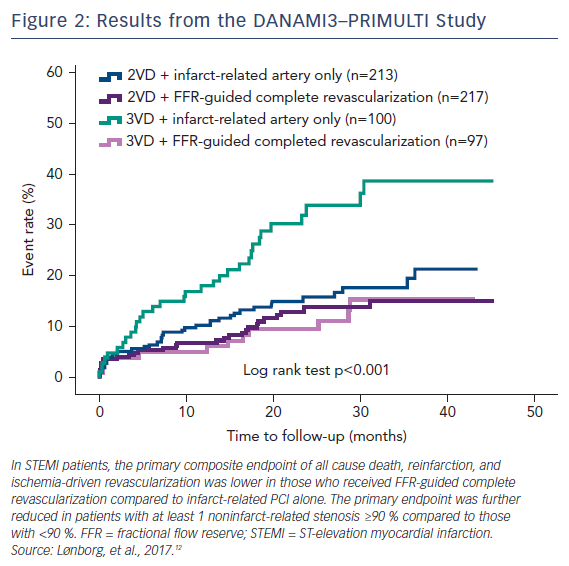
DANAMI3–PRIMULTI was an open-label, randomized controlled trial that enrolled 627 patients with STEMI who had ≥1 clinically significant coronary stenosis in addition to the culprit lesion. After successful PCI to the culprit lesion, patients were randomized into no further invasive treatment or complete FFR-guided revascularization before discharge. A threshold of FFR <0.80 was used, and FFR was performed 2 days after primary PCI to avoid the risk of invalid FFR measurements inferred from acute changes in macrovascular tone or microvascular flow obstruction. The primary endpoint of a composite of all-cause mortality, non-fatal reinfarction, and ischemia-driven revascularization of lesions in noninfarct-related arteries was significantly lower in the complete revascularization group (13 %) compared with the infarct-related only group (22 %). The favorable effect was driven by significantly fewer revascularizations. A substudy of the DANAMI3–PRIMULTI trial found that the benefit of staged FFR-guided complete revascularization was observed primarily in patients with threevessel disease and at least one noninfarct-related stenosis with a ≥90 % diameter (Figure 2).12
In the Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction - COMPARE-ACUTE trial 885 patients with STEMI and multivessel disease who had undergone primary PCI of the infarct-related artery were randomized to undergo FFR-guided complete revascularization of noninfarct-related coronary arteries or no revascularization of noninfarct-related arteries. Unlike DANAMI3– PRIMULTI, FFR of the noninfarct-related artery was done in the acute STEMI setting, during the time of primary PCI. At one year those who underwent FFR-guided complete revascularization of noninfarct-related arteries had a lower risk of death (1.4 % versus 1.7 %), MI (2.4 % versus 4.7 %), revascularization (6.1 % versus 17.5 %), and cerebrovascular events (0.0 % versus 0.7 %) compared with those who were treated for the infarct-related artery only.13 Approximately half of the noninfarctrelated artery lesions that were angiographically significant were not physiologically significant by FFR (≥0.80).
The ideal threshold for FFR in the ACS population has been debated. The FFR threshold of 0.80, which is used to determine functional significance in the stable ischemic heart disease (SIHD) population, was applied to the ACS population in the above studies. Hakeem et al. found that using FFR for clinical decision-making in ACS patients using the standard FFR = 0.80 threshold was associated with a threefold increase in the risk of subsequent MI and target vessel failure compared with SIHD patients, and advised caution in using FFR-derived values for clinical decision-making in patients with ACS. They found that ACS patients had a higher FFR threshold of functional significance, and those with an FFR <0.85 had significantly higher event rates than those with FFR >0.85.14
Post-percutaneous Coronary Intervention Fractional Flow Reserve
Implications and Importance
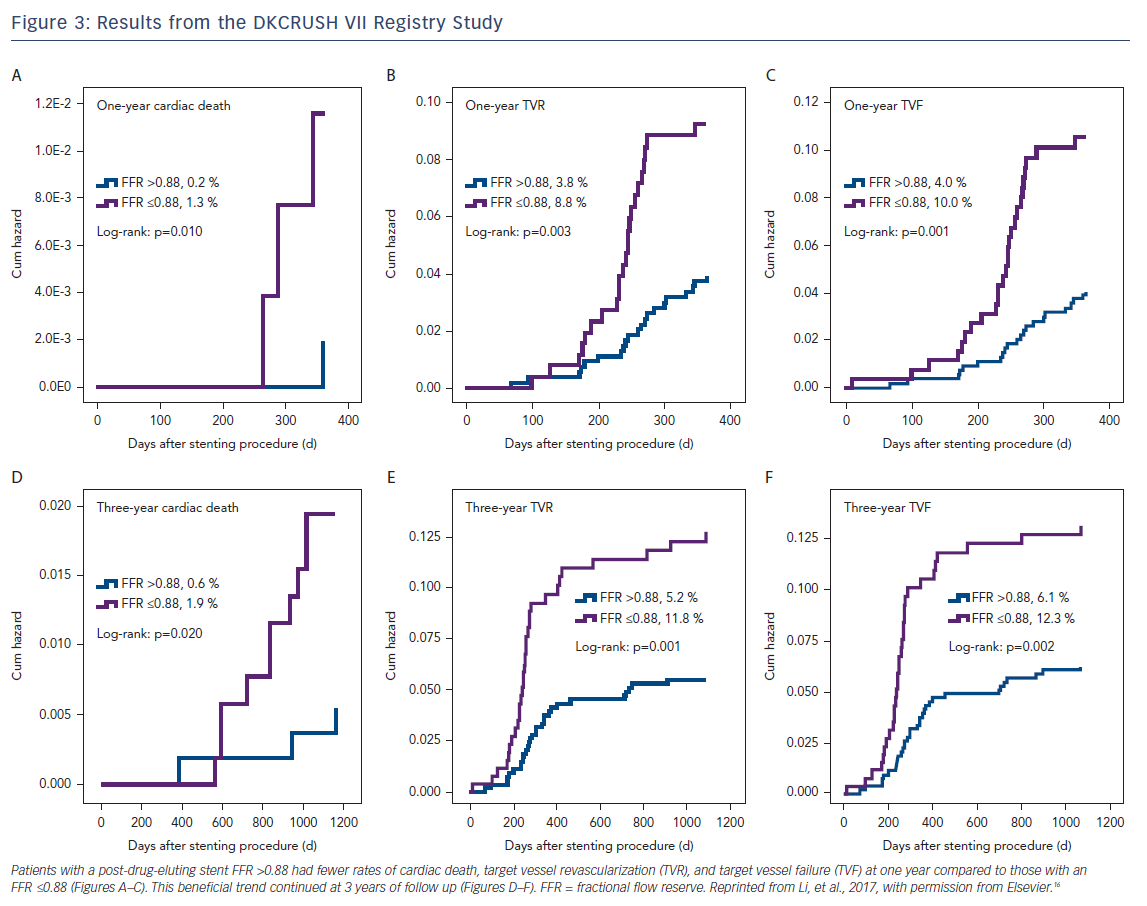
In contrast to pre-PCI FFR, the clinical implications of post-PCI FFR have not been as well demonstrated in large, randomized clinical trials. In a meta-analysis of 105 studies between 1995 and 2005 reporting on post-PCI FFR and evaluating the relationship between post-PCI and clinical outcomes post-PCI, Rimac et al. found that higher post-PCI FFR values are associated with significantly lower risks of repeat PCI and MACE during follow up, and post-PCI FFR values of ≥0.90 are associated with a relative risk reduction of repeat PCI of 55 % and a relative risk reduction of MACE by 30 %.15 Results from the Randomized Study on Double Kissing Crush Technique Versus Provisional Stenting Technique for Coronary Artery Bifurcation Lesions (DKCRUSH VII Registry Study) showed that a post-drug-eluting stent (DES) FFR ≤0.88 strongly correlated with target vessel failure, and this was maintained after 3 years of follow up (Figure 3).16 Impaired post-DES FFR may be influenced by several independent factors including stent length, stent diameter, and disease in the left anterior descending artery. In one study among patients receiving long stents (defined as 30–49 mm), only 12.2 % were found to have an optimal post-PCI defined as an FFR >0.95. Among patients who received ultra-long stents (≥50 mm), none achieved an optimal post-PCI FFR.17
Fractional Flow Reserve and Transcatheter Aortic Valve Implantation for Aortic Stenosis
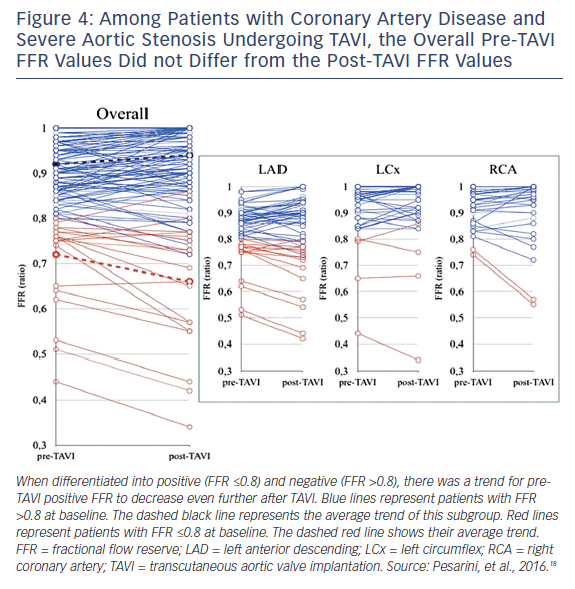
Many patients with severe aortic stenosis (AS) have concomitant CAD, but there are no clearly established guidelines on revascularization management in patients undergoing transcatheter aortic valve implantation (TAVI). The utility of FFR in this patient population has been recently validated. In severe AS there is increased microcirculatory resistance and reduced vasodilatory reserve, which may influence FFR. In a study of 54 patients with severe AS and CAD undergoing TAVI, 154 lesions were assessed by FFR both before and after TAVI. There were no ischemic complications related to the administration of intracoronary adenosine during the FFR procedure or after one month of follow up, demonstrating that an FFR evaluation is safe to perform in severe AS patients. Overall, there were only minor changes in FFR values before and after TAVI, confirming the validity of FFR in this clinical scenario. In approximately 15 % of patients with CAD, the post-TAVI FFR assessment did change the indication to perform PCI, and this was mostly for angiographically intermediate lesions with unmasked functional significance (Figure 4).18
Fractional Flow Reserve and Coronary Flow Reserve
CFR measures flow through both the epicardial resistance conduit and the microcirculation. It is another validated index of the functional significance of a coronary stenosis when the microcirculation is considered normal. In contrast to FFR, reduced CFR has been shown to be a prognostic marker of adverse outcomes in patients with and without epicardial disease. Pre-PCI CFR, and not post-PCI FFR, was shown to be an independent predictor of adverse cardiovascular events after PCI in one study.19
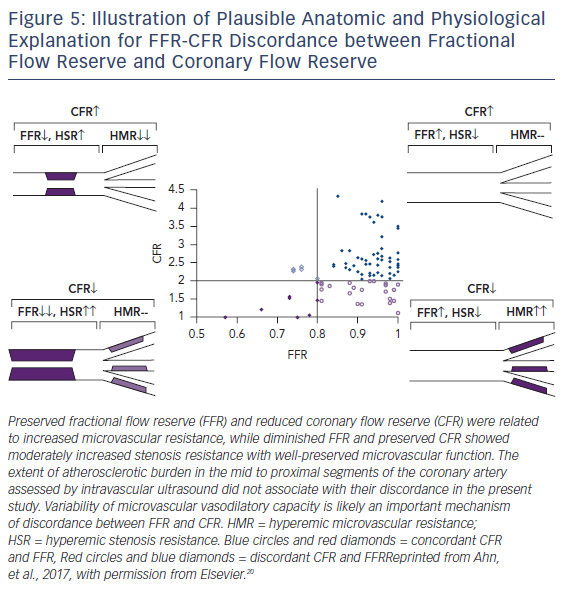
FFR and CFR values may be discordant, even in the same patient. The reason for discordance is not entirely clear (Figure 5). The amount of atherosclerotic burden may influence discordance. In a study of 94 patients with moderate coronary stenosis, intravascular ultrasound was used to measure degree of atherosclerosis. Among patients with preserved FFR, those with low CFR had similar plaque burden and higher microvascular resistance compared with those with preserved CFR. In patients with low FFR, those with preserved CFR had smaller plaque burden and lower microvascular resistance than those with low CFR.20
Instant Wave-free Ratio Outcomes
iFR is a resting index used to assess severity of an intracoronary stenosis. It measures the ratio of Pd to the Pa during an isolated period of diastole (i.e. the “wave-free period”). It is an attractive alternative to FFR because it does not require hyperemia, and therefore has a lower incidence of patient discomfort, side effects, and shorter procedural time.
iFR has been shown to be non-inferior compared to FFR in two large, multicenter, randomized controlled trials. The Instantaneous Wave-free Ratio Versus Fractional Flow Reserve in Patients with Stable Angina Pectoris or Acute Coronary Syndrome (iFR-SWEDEHEART) trial included 2037 patients with stable angina or ACS, and randomly assigned them to undergo either iFR or FFR-guided revascularization. The rate of primary endpoint (composite of death from any cause, non-fatal MI, or unplanned revascularization within 12 months after the procedure) was not significantly different between the two groups.21 In the Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularisation (DEFINE-FLAIR) trial, 2492 patients with CAD were randomized to have iFR-guided or FFR-guided coronary revascularization. The primary endpoint of a composite of death from any cause, non-fatal MI, or unplanned revascularization did not differ significantly between groups. Additionally, the number of patients in the iFR group had lower rates of adverse side effects from the procedure (3.1 % versus 30.8 %), and a shorter median procedural time (40.5 minutes versus 45.0 minutes) compared with the FFR group.22
Comparisons of iFR and FFR remain a hot topic. Critiques of iFRSWEDEHEART and DEFINE-FLAIR include the low-risk populations studied and the large non-inferiority margins used. The safety of using iFR instead of FFR in patients with higher-risk (i.e. more severe ischemic lesions with low FFR) CAD is debatable as the average FFR in the two iFR studies was much higher than in FAME trials (FFR 0.83 versus 0.71). While IFR and FFR are generally concordant 80 % of the time, especially for severe and minimal stenoses, iFR and FFR discordance occurs in about 20 % of patients and raises a clinical decision-making dilemma – Which one is superior? That question is an ongoing area of active research.
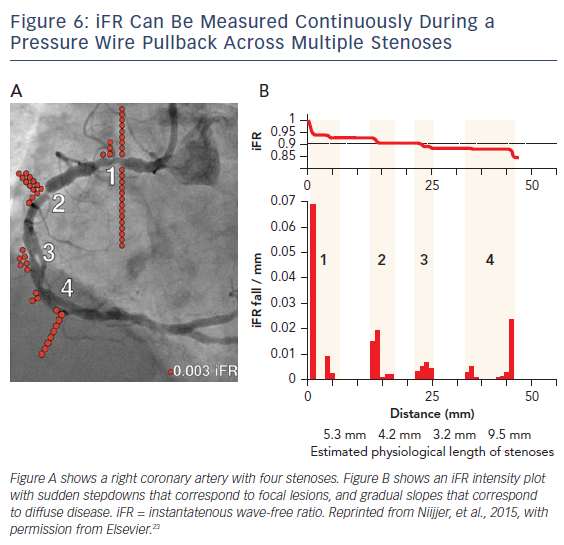
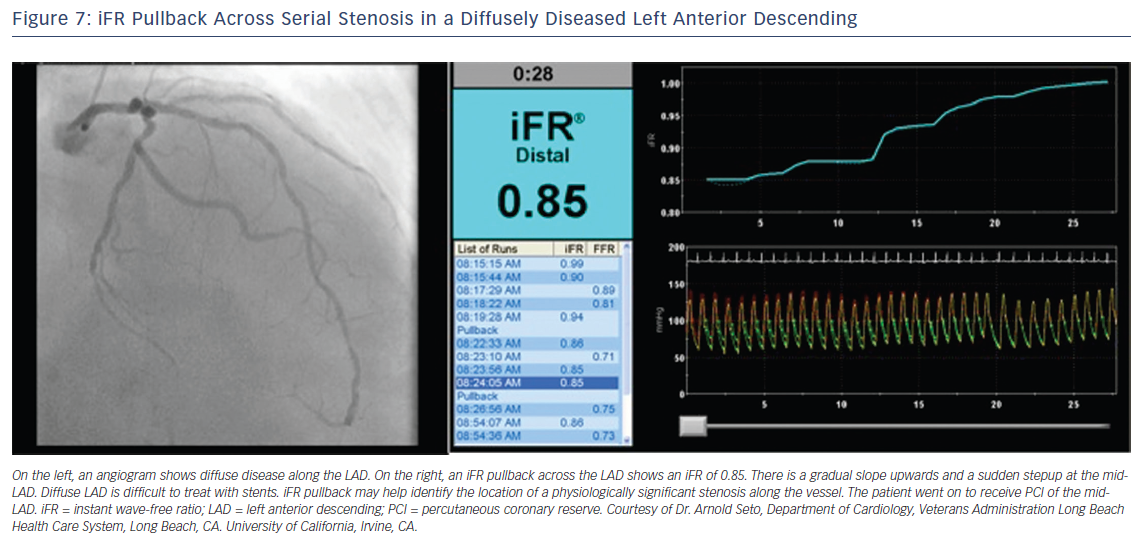
iFR has the potential to simplify treatment of serial lesions by pullback iFR measurements co-registered with the angiographic lesion locations (Figures 6 and 7).23 This technology will likely be a vanguard of future research into the interaction of resting and hyperemic flow across serial stenoses.
Conclusion
Coronary physiology is now recognized as a key part of the clinical decisionmaking for interventional cardiologists. Exciting developments over the past few years have led to a renewed interest in the field. With strong data showing favorable outcomes associated with the use of measuring coronary physiology in the cardiac catheterization lab, especially FFR and iFR, the days of angiography alone-based PCI are over. The future lies in new methods of physiologic measurements including iFR, CFR, indices of microvascular resistance, index of microvascular resistance, and expanding the use of these methods to unique clinical scenarios.