Transcatheter aortic valve replacement (TAVR) has become the preferred method for management of severe aortic stenosis in patients who are at high and intermediate surgical risk. Short-term data have been presented from two studies comparing TAVR versus surgical aortic valve replacement (SAVR) in low surgical risk patients. The Placement of Aortic Transcatheter Valves 3 (PARTNER 3) trial compared TAVR using a balloon expandable valve with standard SAVR. The primary endpoint was a composite of all-cause mortality, stroke, or re-hospitalization at 1 year post-procedure. There was a near 50% reduction in the primary endpoint in the TAVR group.1 The Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients (EVOLUT) trial showed similar results using a self-expanding platform. The primary endpoint of all-cause mortality or disabling stroke at 2 years was similar in the TAVR and SAVR groups, meeting criteria for non-inferiority.2
These landmark trials indicate that TAVR will soon become standard of care for a much larger patient population in the US, adding to the need for more data about peri-TAVR care, including pharmacological management to reduce the risk of thrombotic and bleeding complications associated with the procedure. Specifically, there is a gap in the evidence supporting the current guideline-mediated therapy for antithrombotics post-TAVR. Thrombocytopenia in patients undergoing TAVR is associated with increased mortality and morbidity. The poorly understood phenomenon forms a fertile ground for future research.
In this article, we review current guidelines for antithrombotic therapy in patients undergoing TAVR, studies evaluating antiplatelet regimens, and studies evaluating the use of platelet function testing following TAVR. We also offer a potential link between thrombocytopenia and antiplatelet treatments in patients undergoing TAVR.
TAVR-associated Thrombocytopenia and Thrombotic Events
About 40–70% of patients undergoing TAVR develop thrombocytopenia, and the proportion can be as high as 87%.3,4 Additionally, post-TAVR patients experience an average decrease in platelet count by about 40% from baseline.5 Severe thrombocytopenia, defined as a platelet count ≤50 × 109 /l , is an independent risk factor for long-term mortality post-TAVR, as it is associated with higher rates of both strokes and major bleeding events.6 Dvir et al. found 1-year mortality rates in patients with severe thrombocytopenia post-TAVR ranged from 20–67%, a vast increase from baseline rates in patients with no or mild thrombocytopenia.5
Platelet count reaches a nadir 2–3 days after TAVR and spontaneously recovers by days 5 and 6 after the procedure. While the exact mechanism of thrombocytopenia is not known, the spontaneous recovery suggests a consumptive mechanism by means of potential platelet activation and aggregation at the level of the valve prosthesis.3,7 Another potential mechanism is heparin-induced thrombocytopenia (HIT). However, Jilaihawi et al. found no evidence that either HIT or bioprosthetic function contributed to early major or persistent thrombocytopenia. Additionally, there was no association between TAVR approach and the magnitude of post-TAVR thrombocytopenia.3 New data suggest that P2Y12 inhibition prior to the procedure may blunt the severity of TAVR-associated thrombocytopenia.8
In‐hospital and early thromboembolic event rates after TAVR range between 2–4%.9 More than 70% of these events occur within 2 days of the procedure.10 Data from studies investigating cerebral protection devices have demonstrated that acute thrombi, rather than calcified or valvular debris, were the most frequently captured material by these devices (~99%).11 It is conceivable that platelets are being consumed in forming these acute thrombi. In addition to strokes, new imaging techniques have also led to improved visualization of the recently described phenomenon of hypoattenuated leaflet thickening (HALT).12 It is speculated that this reflects a process invoked by the inflammatory milieu triggered during valve replacement that causes platelet activation on the surface of the prosthetic valves. It may be the case that this thromboinflammatory reaction to valve prostheses is the inciting factor for perioperative thrombocytopenia, though this understudied area is in need of greater investigation.7 The prevalence of HALT in TAVR patients was found to be 18% in a study by Nührenberg et al. looking at the effects of dual anti-platelet therapy (DAPT) on the prevention of subclinical leaflet thrombosis.13 While the study found that rates of symptomatic thrombosis occurred in only 1–2% of patients, it remains unclear if the high rates of subclinical HALT portend adverse long-term clinical outcomes.13 In one study with longitudinal follow-up after TAVR, patients with HALT had lower platelet counts at discharge, 6 months and 1 year after TAVR compared with those without HALT.14
While the majority of patients undergoing TAVR develop thrombocytopenia, the exact mechanism of thrombocytopenia after TAVR has not been elucidated. Additionally, it is unknown whether or not this phenomenon is associated with increased platelet turnover (i.e. higher levels of immature platelets). It is well-documented that immature platelets are more active than senescent platelets in thrombus formation. Changes in the von Willebrand factor (vWF) mutimeric structure during and shortly after TAVR may offer a possible explanation for TAVR-associated thrombocytopenia. Von Willebrand factor is the major circulating macromolecule that regulates platelet adhesion and aggregation in response to shear forces.15 High molecular weight multimers of vWF have the highest affinity to bind to platelets.16 It is conceivable that the recovery of these multimers is associated with increased interaction with platelets during and shortly after TAVR, resulting in platelet clumping and a decrease in platelet counts. In vitro studies have shown that rapid phosphorylation of adenosine diphosphate and P2Y12 blockade inhibits shear-induced platelet aggregation, a phenomenon thought to cause unfolding of vWF.17 This interaction appears to be resistant to aspirin.18 Hence, aspirin monotherapy is unlikely to have any effect on TAVR‐associated thrombocytopenia.
Platelet Reactivity and Thrombotic Events in Trancatheter Aortic Valve Replacement
Two relatively unexplored areas in the debate surrounding antiplatelet and antithrombotic regimens in people who have had TAVR are the significance of high platelet reactivity (HPR) and whether or not HPR and response to DAPT correlates with the incidence of HALT. Given the correlation between HPR and increased risk of post-percutaneous coronary intervention (PCI) ischemic events, some researchers have sought to clarify the role of HPR specifically in patients undergoing TAVR.19 Other studies have explored the relationship between platelet reactivity, antiplatelet drugs, and the incidence of HALT, with variable results.
One of the first studies in this area is the Assessment of Platelet REACtivity After Transcatheter Aortic Valve Replacement trial, a randomized, prospective multicenter study investigating platelet reactivity profiles among patients undergoing TAVR treated with acetylsalicylic acid (ASA) and clopidogrel compared with those with HPR placed on ASA and ticagrelor.20 Data from 59 patients were included. Extrapolated from the PCI population, a cut-off value of 208 P2Y12 reactivity units was used to define HPR. More than two-thirds of the 68 participants (n=48) had HPR on clopidogrel at baseline, a prevalence slightly higher than has been described in PCI data among elderly patients (≥75 years).21,22 Unexpectedly, more than one-third of patients who responded to clopidogrel at baseline were found to have HPR 30 days post-TAVR. This observation raises questions about physiologic changes occurring post-TAVR that may contribute to the increased incidence of HPR beyond that seen in PCI data. Of note, ticagrelor showed highly effective suppression of platelet activity, with <10% HPR at 6 hours and 100% responders at 5 days, which remained consistent throughout the 3 months. The sample size was too small to allow any conclusions regarding ticagrelor’s safety in this population.
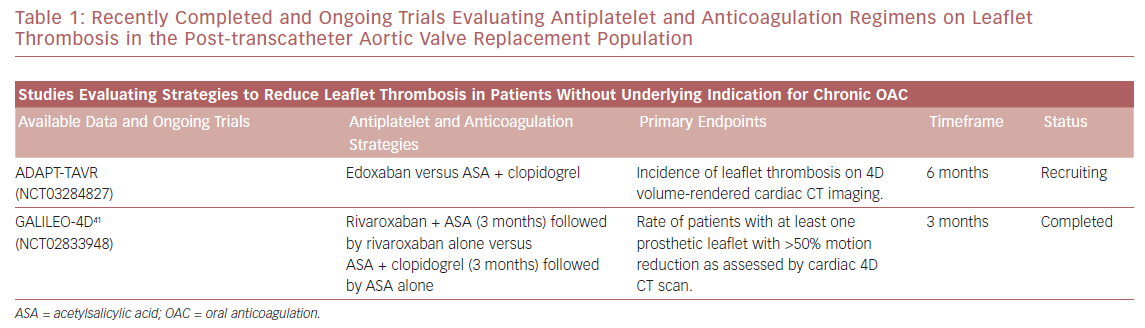
However, a separate study by Nührenberg et al. concluded that DAPT had no effect on the rates of HALT seen post-TAVR, regardless of clinical outcomes.13 This was a study of all-comers, including those with a prior indication for oral anticoagulation (OAC), who were placed on either DAPT with ASA plus clopidogrel or OAC plus clopidogrel. Platelet function testing was performed at the start of the procedure and patients were evaluated using 4D CT 5 days after valve implantation to assess the association between baseline platelet reactivity and HALT. HPR was defined as >468 aggregation units × minute. The investigators found no relationship between HPR and incidence of HALT, with considerable risk for HALT even among subjects who responded well to clopidogrel. Ongoing studies to elucidate the optimal pharmacological therapy to reduce leaflet thrombosis are listed in Table 1 .
The main concern with studies evaluating HPR in TAVR patients is the extrapolation of HPR cut-off values from PCI populations. Although it is well-established that HPR correlates with ischemic events, including stent thrombosis, measuring platelet reactivity to guide clinical decisions is no longer routine practice during or after PCI in the era of newer generation drug-eluting stents. Furthermore, thrombotic events in the TAVR population may have a different mechanistic explanation. Most notably, the role of vWF has not been established in the PCI population, as well as platelet activation and aggregation on the valve surface.23 Dedicated on-treatment platelet reactivity studies in TAVR patients are needed. Those studies should focus on identifying HPR cut-offs that correlate with TAVR-specific ischemic events (stroke and HALT).
While Nührenberg et al. concluded that DAPT has no effect on the rates of HALT post-TAVR, certain patient factors were associated with impaired response to antiplatelet therapy, as measured by a high on-treatment PR, leading to increased risk of thrombotic and ischemic events. These factors included advanced age, diabetes, polypharmacy, and low BMI.24 Considering that the current population of TAVR recipients are those patients at intermediate and high risk of surgical complications, they are more likely to exhibit factors that alter response to antiplatelet therapy. In addition, the current TAVR population is also at high risk of major bleeds, at rates of up to 16% in the first year.25
The decisions surrounding antithrombotic management are further complicated by the large proportion of patients undergoing TAVR who require anticoagulation for concomitant AF, which is seen in about 30% of the TAVR population.26 While current post-TAVR antithrombotic recommendations support DAPT unless prior OAC is indicated, the evidence to support this practice is limited. A 2019 study of patients with AF and CHA2 DS2 -VASc (congestive heart failure, hypertension, age ≥65 or ≥75 years, diabetes, stroke/transient ischemic attack, vascular disease, sex category) score ≥2 from the PARTNER 2 cohort showed a decreased rate of stroke at 2 years post-TAVR in patients on antiplatelet therapy (single or dual) even without OAC.27 The study did not, however, address the addition of an antiplatelet to anticoagulation versus anticoagulation alone. Several ongoing studies discussed below seek to clarify the most appropriate regimens.
Antithrombotic Therapy Before and During Transcatheter Aortic Valve Replacement
The main question before a TAVR procedure is whether or not to preload patients with P2Y12 inhibitors. Pre-procedural P2Y12 loading was found to decrease the severity of thrombocytopenia in a single-center study.7 Whether pre-procedural P2Y12 inhibition has any effect on clinical events remains to be determined. As far as intraprocedural antithrombotic management, unfractionated heparin (UFH) remains the gold standard. In the randomized open-label, Bivalirudin Versus Heparin Anticoagulation in Transcatheter Aortic Valve Replacement Phase 3 trial, 802 patients at high surgical risk who were scheduled for transfemoral TAVR were randomized to receive either bivalirudin (n=404) or UFH (n=398). The trial was designed to show superiority of bivalirudin in regard to bleeding events. The co-primary endpoints were major bleeding (defined as Bleeding Academic Research Consortium type ≥3b), either within 48 hours or before hospital discharge, whichever occurred first, and 30-day net adverse cardiovascular events (NACE). Bivalirudin for procedural anticoagulation did not significantly reduce the primary outcomes. At 30 days, NACE rates were 14.4% in the bivalirudin group and 16.1% in the UFH group (p=0.50).28
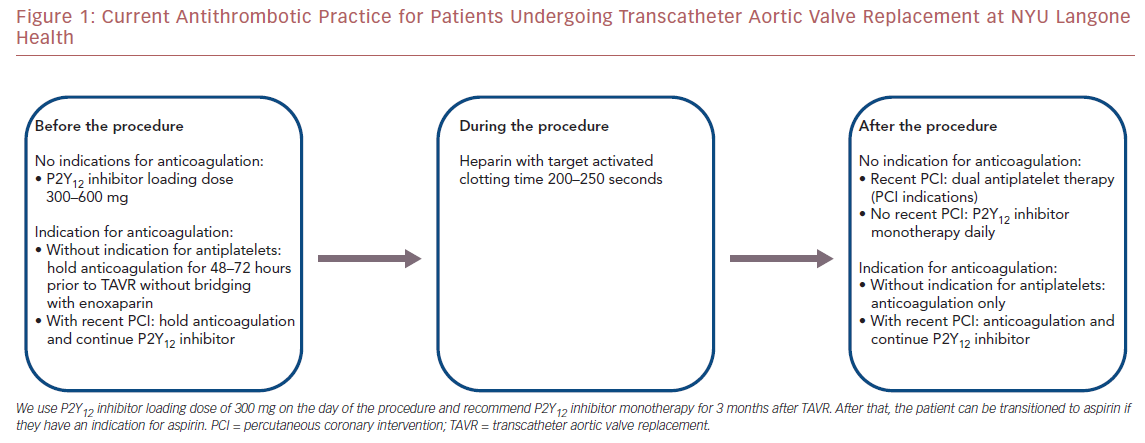
Figure 1 shows the current antithrombotic practice at our center based on the available data. We use a clopidogrel loading dose of 300 mg on the day of the procedure. We recommend clopidogrel monotherapy for 3 m onths after TAVR. After that, the patient can be transitioned to aspirin if they have an indication for aspirin.
Current Antithrombotic Recommendations after Transcatheter Aortic Valve Replacement
The current guidelines for antithrombotic management peri-TAVR have been extrapolated from data obtained from other areas in the field, namely in a post-PCI population. The data behind the use of DAPT versus other antithrombotic regimens specific to TAVR patients are limited.
Guidelines from the American College of Cardiology and the American Heart Association recommend DAPT with aspirin and clopidogrel for 6 months unless there is another indication for OAC, followed by lifelong aspirin monotherapy (class IIb recommendation based on level C evidence). The European Society of Cardiology and the European Association for Cardio-Thoracic Surgery similarly recommends 3–6 months of DAPT followed by lifelong single antiplatelet therapy (IIa–C). The Canadian Cardiovascular Society guidelines suggest treating with low-dose aspirin along with 1–3 months of a thienopyridine based on consensus of expert opinion. The evidence behind these guidelines is based on empiric treatment in the initial clinical trials’ protocols for TAVR, but to date, DAPT has not demonstrated benefit in larger randomized trials.29,30
Available Data and Limitations
As mentioned previously, many of the current recommendations and guidelines surrounding antithrombotic strategies in TAVR patients are based on empiric regimens and extrapolated from studies looking at patients undergoing PCI, as well as initial trials comparing TAVR with SAVR. With new data from PARTNER 3 and EVOLUT suggesting benefits of TAVR in low-risk patients, there is an even greater need to clarify appropriate treatment and potentially alter current practices based on a changing patient population.
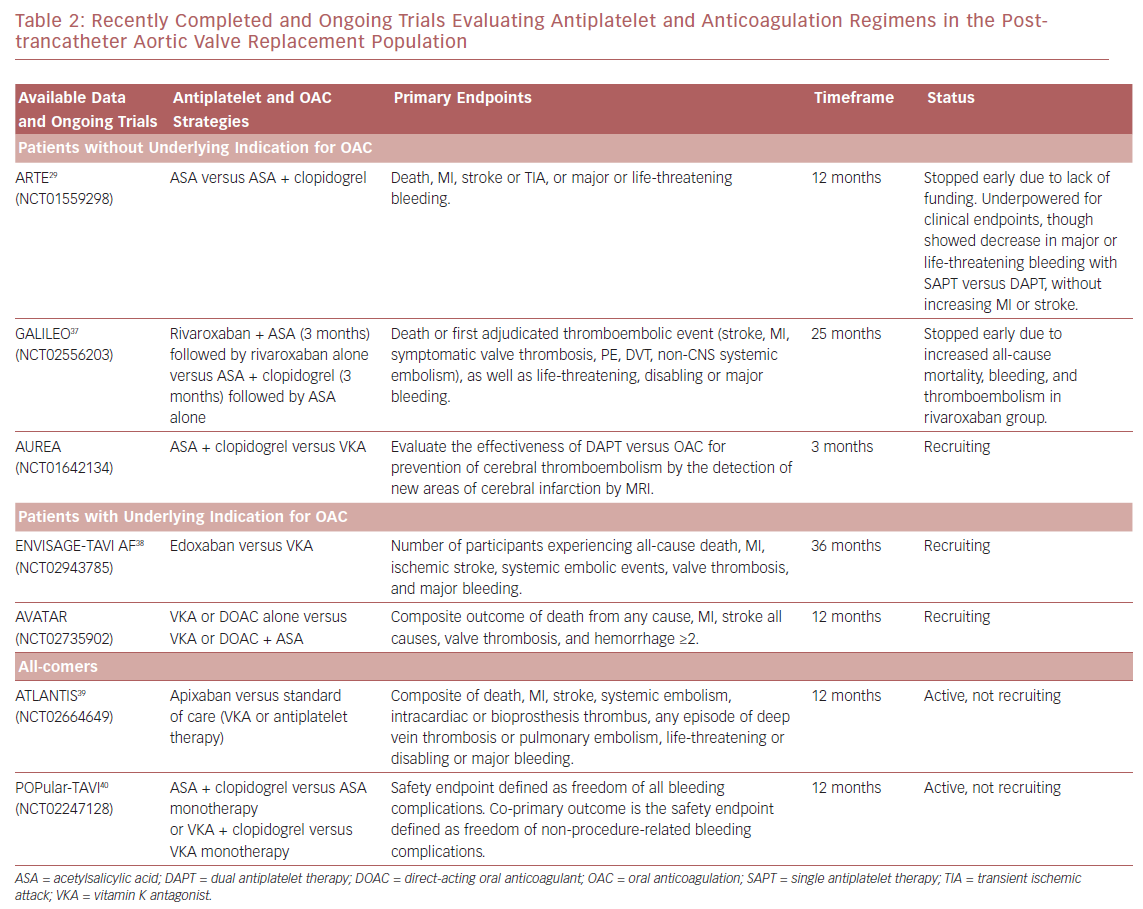
There are multiple ongoing clinical trials investigating different antithrombotic regimens in patients undergoing TAVR (Table 2 ). In the Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation (ARTE) trial, aspirin monotherapy was compared with DAPT, though the trial was terminated early due to a greater number of major or life-threatening bleeding events in the DAPT arm (10.8% versus 3.6%; p=0.038).29 These event rates were similar to the bleeding event rates seen in the 2011 PARTNER trial, with major bleeding occurring in 9.3% of subjects (32 of 348) at 30 days, all of whom received DAPT.31 With the data obtained from the 222 patients enrolled in the ARTE trial, single antiplatelet therapy (SAPT) had lower rates of major bleeding while showing no increase in risk for MI or stroke. However, this small study was underpowered for clinical endpoints and larger randomized trials are needed to substantiate these results.
In a 2017 meta-analysis by Verdoia et al. looking at safety and efficacy of antithrombotic strategies in post-TAVR patients, DAPT did provide an overall mortality benefit (12.2% versus 14.4%; OR, 0.81) compared with SAPT.32 Additionally, DAPT showed slight benefit compared with SAPT for stroke. However, the similar rates of bleeding between the DAPT and SAPT arms call into question the validity of the study results. This meta-analysis supports the current use of DAPT post-TAVR with SAPT plus OAC reserved for patients with prior indications for anticoagulation. However, there are several limitations to this study mostly related to the small size of the studies included in the meta-analysis, as well as the non-randomized design. Additionally, the majority of patients involved in the study were placed on DAPT (>70%) with the remainder receiving SAPT ± OAC, leading to a possible amplification of the benefits associated with this treatment. Given the lack of randomization of these studies, it is also possible that the SAPT arm included a disproportionate number of patients who had greater frailty or were at greater risk of adverse events than those placed on DAPT. Finally, there were inconsistent long-term data from the individual studies, with some only providing in-hospital events, potentially missing or under-reporting events after hospitalization.
Other studies that have tried to further elucidate the best antithrombotic regimens post-TAVR include those looking at various antiplatelet combinations, as well as those investigating anticoagulation versus antiplatelet agents. In the Global Study Comparing a rivAroxaban-based Antithrombotic Strategy to an antipLatelet-based Strategy After Transcatheter aortIc vaLve rEplacement to Optimize Clinical Outcomes (GALILEO) trial, anticoagulation regimens were compared with or without the addition of an antiplatelet agent. Specifically, rivaroxaban plus aspirin was compared with standard DAPT treatment. This trial was prematurely stopped due to an increase in all-cause mortality in the rivaroxaban group (6.8% versus 3.3%), as well as increased bleeding (4.2% versus 2.4%) and thromboembolism (11.4% versus 8.8%).33,34 Although there is a lack of publicly available data after the termination of this study, one possible explanation for the increase in bleeding and thromboembolism in this case is the highly frail TAVR population who is at increased risk of both bleeding and thrombosis. Additionally, the increased mortality rate most likely mirrored the increase in bleeding events.
One study that focused on patients with an indication for anticoagulation compared anticoagulation using warfarin only with anticoagulation plus SAPT and DAPT in patients undergoing TAVR. During 13 months of follow-up, patients who received warfarin and any combination of antiplatelet therapy had higher rates of major and life-threatening bleeding with no significant reduction in thrombotic events.35 However, another study by Kosmidou et al. showed ischemic benefit of adding antiplatelets to oral anticoagulation after TAVR. The main issue with this study is that patients with AF who received antiplatelets alone had lower rates of stroke than those who received anticoagulation alone (4.2% versus 8.3%), which contradicts the known superiority of anticoagulation in preventing strokes in these patients.27
Furthermore, one recent study found no difference in bleeding events between warfarin and non-vitamin K oral anticoagulants (NOACS) in terms of bleeding events.36 These studies have been the basis of our strategy to omit antiplatelet agents in patients undergoing TAVR who require anticoagulation. At our center, patients who are on any anticoagulant without indications for antiplatelets do not receive any antiplatelet drugs before or after TAVR. The choice of anticoagulation is discussed with each patient individually, given there are no major differences between warfarin and NOACs (Figure 1 ).
Future Directions
The field of antithrombotics in TAVR patients is still in its infancy. The optimal antithrombotic regimen in patients undergoing TAVR is yet to be identified. The rationale for DAPT following TAVR is to decrease the risk of thromboembolic events associated with this procedure, especially cerebrovascular events.26 This issue is further complicated with about 30% of patients undergoing TAVR requiring anticoagulation for concomitant AF. Recent studies have suggested that a single antiplatelet drug after TAVR may be as effective with a better safety profile. However, which antiplatelet drug is still to be determined.
Studies evaluating SAPT versus DAPT have conventionally investigated aspirin in the SAPT arm.29 The fact that P2Y12 inhibition appears to blunt the severity of thrombocytopenia in patients undergoing TAVR may offer P2Y12 monotherapy as an alternative to DAPT and to aspirin monotherapy in these patients. This is particularly useful in the era of shrinking indications of aspirin for primary prevention of cardiovascular disease, especially in those aged ≥70 years, where aspirin is no longer recommended for primary prevention.
Whether patients with an indication for anticoagulation should receive one, two, or any antiplatelet therapies at all remains to be determined in large studies. Although one recent study showed ischemic benefit of adding antiplatelets to anticoagulation in patients with AF undergoing TAVR, other studies suggest that warfarin-only therapy may be safer and as effective as warfarin with one or two antiplatelet agents.27,35 While no difference in terms of bleeding events was seen between warfarin and NOACs, it appears that anticoagulation with rivaroxaban for all TAVR patients carries more harm than benefit, which lead to the termination of the GALILEO trial.36 With a rapidly expanding TAVR population, there is a pressing need for research into the pathophysiology of the thrombocytopenia associated with TAVR and the optimal antiplatelet strategies to minimize the risk of subsequent thrombosis. Prospective studies are also needed to determine the best antithrombotic combination for patients with AF who undergo TAVR. Finally, the development of bleeding risk scores that are specific to TAVR population and tailoring antithrombotic therapy based on individual bleeding risk, the safety and efficacy of intraprocedural intravenous P2Y12 inhibition, and the use of platelet turnover markers (i.e. immature platelet levels) to guide antithrombotic therapy in patients undergoing TAVR all require investigation.