Chronic total occlusions (CTOs) are a common angiographic finding in patients with coronary artery disease (CAD). According to the findings of a Canadian multicenter registry, the prevalence of CTOs is approximately 18% in patients with CAD undergoing coronary angiography and 10% in those presenting with ST-elevation MI (STEMI), reaching levels as high as 89% in patients with prior coronary artery bypass grafting (CABG).1,2 Robust data, mainly from observational studies, but also randomized studies, have shown beneficial effects of successful percutaneous coronary intervention (PCI) in people with CTO in terms of symptom improvement – the main indication for CTO recanalization.3–7 Furthermore, multiple observational studies report successful CTO PCI to be associated with higher survival and lower mid- and long-term major adverse cardiovascular event (MACE) rates. However, survival benefit has not been replicated in randomized controlled trials.8–12 On the other hand, CTO interventions are considered to be high-risk procedures, having a substantial rate of acute and late complications. The insertion of a variety of devices in the coronary artery tree, such as the numerous microcatheters and guidewires frequently used during a long-lasting CTO PCI, predisposes to thrombus formation, with rates of acute stent thrombosis reported to be up to 2%.13 Likewise, the presence of extensive calcification – a common characteristic of CTO lesions – hinders stent expansion leading to both acute and late in-stent thrombosis.14 Hence, it is clearly understood that optimal antithrombotic therapy during the peri- and post-procedural period is a key component in achieving and maintaining a successful PCI outcome.
Periprocedural Intravenous Anticoagulation and Antiplatelet Therapy
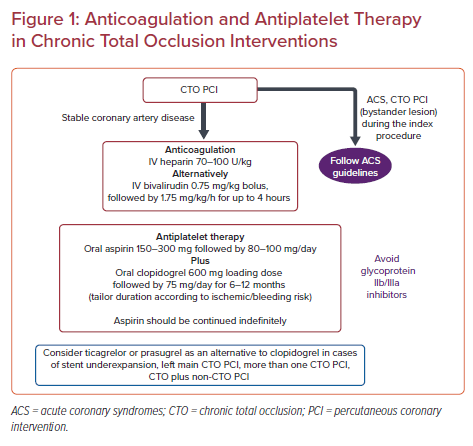
According to the recent European guidelines on myocardial revascularization, unfractionated heparin (UFH) is the standard anticoagulant during elective PCI (70–100 U/kg) with bivalirudin (0.75 mg/kg bolus, followed by 1.75 mg/kg/h for up to 4 hours after the procedure) mainly being used in cases of heparin-induced thrombocytopenia. Enoxaparin (IV 0.5 mg/kg) should be considered as an alternative agent.15 Focusing on CTO interventions, in theory, UFH is the preferred anticoagulant agent having the advantage over bivalirudin that it can be reversed with the administration of protamine in cases of severe perforation. Moreover, there are unpublished cases in which guide catheter thrombosis occurred during long-lasting procedures when bivalirudin had been used.16
On the other hand, bivalirudin can be used as an alternative in patients with heparin-induced thrombocytopenia. Nevertheless, randomized data are encouraging regarding the perioperative administration of bivalirudin in CTO PCI. A single-center pilot study of 84 patients at high bleeding risk who underwent CTO PCI showed no difference in in-hospital MACE (defined as the composite of all-cause mortality, cardiac death, stent thrombosis, periprocedural MI, or additional unplanned target lesion revascularization, or any other post-PCI ischemic event; 21.4% versus 14.3% for bivalirudin and heparin respectively; p=0.393) and major bleeding events (4.8% versus 9.5%; p=0.676). For both groups of the study the activated clotting time (ACT) was aimed to be ≥300 seconds.17 Likewise, another single-center prospective randomized controlled trial recruited 123 elderly CTO patients at high bleeding risk. The in-hospital MACE rate was similar between bivalirudin and UFH (17.6% versus 20%; p=0.82), as well as Bleeding Academic Research Consortium (BARC) type 1–2 bleeding events (8.8% versus 10.9%, P=0.77) with no BARC type 3–5 bleeding events in either group.18 Mean ACT in the bivalirudin group was 356.6 seconds. It is worth noting that a glycoprotein IIb/IIIa receptor inhibitor was administered in 18.7% of the patients in the UFH group compared with none in the bivalirudin group. In another randomized control trial that included 74 patients with CTO lesions, bivalirudin was associated with a lower incidence of perioperative bleeding (5.6% versus 23.7%; p=0.028) and slow-flow/no-flow (0.0% versus 15.8%; p=0.025) suggesting that bivalirudin may even be safer than UFH for CTO interventions.19

Nevertheless, the studies described here had a small sample size and were likely underpowered, so their results should be interpreted with caution. Furthermore, the higher incidence of perioperative bleeding with heparin that was found in one study was potentially – at least partially – associated with the access site. The increase of radial usage in CTO interventions that has been observed over time may attenuate these differences.20 In addition, the significantly higher cost of bivalirudin is another factor that should be taken under consideration for the selection of anticoagulation therapy. Studies comparing UFH with bivalirudin administration in CTO interventions are summarized in Table 1.
The recommended ACTs are >300 seconds for antegrade CTO PCI and >350 seconds for retrograde CTO PCI, with some operators aiming for >300 seconds with frequent checking if it is in the low 300 seconds range. These recommendations are based mainly on expert opinion rather than data derived from studies. ACT should be checked every 20–30 minutes depending on how high above the target ACT the most recent measurement was. Furthermore, some operators administer a heparin drip, in addition to the initial bolus, to avoid significant fluctuation in anticoagulation levels.16 Any contamination of the blood specimen with water, contrast agent, or drugs may strongly influence the result and thus should be strictly avoided.
Protamine can be used to reverse the action of heparin. To neutralize heparin, 1 to 1.5 mg of protamine is injected per 100 units of heparin (max dose 50 mg) administered at a rate not exceeding 5 mg per minute. Follow-up doses of protamine of 0.5 mg per 100 units of heparin can be given if bleeding continues 4 hours later. Nevertheless, it should be highlighted that protamine is only used as a last resort in cases of perforation – and only after meticulous efforts to control extravasation by other means such as covered stents or coils/fat have failed – since it can cause thrombosis of the equipment that is still in the patient’s arterial tree.
Administration of glycoprotein IIb/IIIa inhibitors should be, in general, avoided during CTO PCI, even after successful crossing and stenting, as it may cause an unrecognized perforation to bleed, leading eventually to tamponade. Furthermore, their use has been independently associated with an increased risk for death (OR 32.29; 95% CI [6.03–172.75]) in a national registry.21 Interestingly, a case of recanalization of a bystander CTO at a subsequent angiography has been reported in a patient who was administered eptifibatide and dual antiplatelet therapy (DAPT) during primary PCI for a STEMI.22 The authors speculated that excessive antithrombotic and anticoagulant therapy aided the intrinsic fibrinolytic mechanism to dissolve the CTO lesion. Nevertheless, the administration of glycoprotein IIb/IIIa inhibitors may be needed in the presence of donor vessel thrombosis during a retrograde crossing attempt.
In the event of bleeding complications after abciximab administration, platelet transfusion can at least partially reverse inhibition of platelet aggregation.
Dual Antiplatelet Therapy
Aspirin (150–300 mg orally or 75–250 mg IV followed by 80–100 mg/day) and clopidogrel (600 mg oral loading dose and 75 mg maintenance dose) administration for 6 months followed by indefinite administration of aspirin is considered the standard of care after PCI in the clinical context of stable CAD. Most of the time, CTO PCI is performed as a scheduled non-ad hoc intervention in patients who present with symptoms related to stable CAD, such as walking angina or exertional dyspnea, thus aspirin and clopidogrel is the standard dual antiplatelet regimen initiated. In patients who present with an acute coronary event and also have a bystander CTO lesion that is recanalized during the index PCI of the culprit lesion or at a subsequent intervention, a more potent P2Y12 receptor inhibitor (prasugrel, ticagrelor) should be used instead of clopidogrel (Figure 1).
The contemporary ISAR-REACT 5 trial showed benefit with prasugrel over ticagrelor in terms of fewer MIs, thus it seems reasonable to be the first option in the clinical context of acute coronary syndrome.23 In the clinical setting of stable CAD, the duration of DAPT can be tailored accordingly to less or more than 6 months after balancing ischemic and bleeding risk. Optical coherence tomography (OCT) studies have shown that CTO PCI is related with delayed stent coverage and a high incidence of malapposition (both associated with stent thrombosis) justifying prolonged DAPT administration.24,25 In the 2017 European Society of Cardiology focused update on dual antiplatelet therapy in CAD, CTO PCI falls under the umbrella of complex PCI, thus prolonged (>6 months) DAPT therapy is recommended after CTO interventions (Class IIb, level of evidence B).26
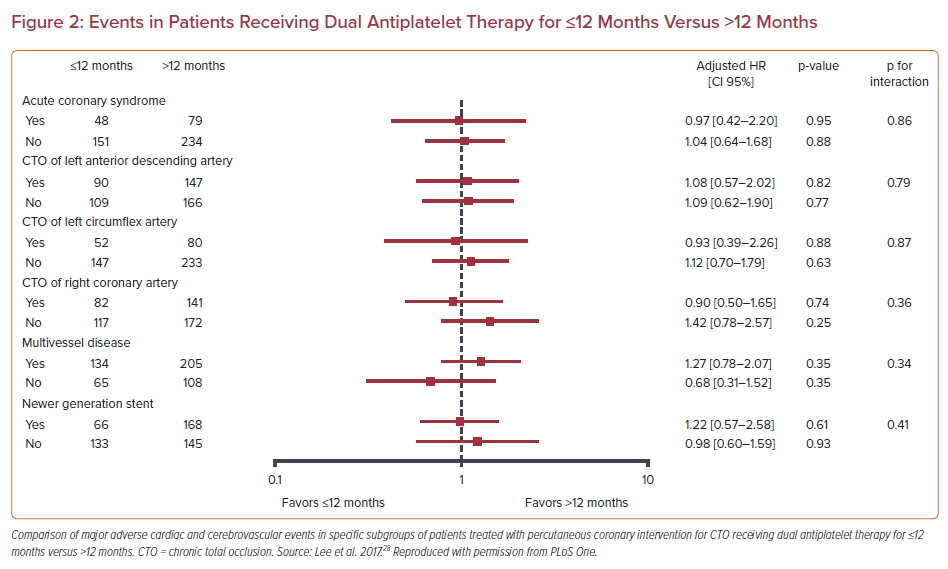
Two recent studies failed to show any benefit from the extended DAPT administration in patients who underwent CTO PCI. Giustino et al. sought to investigate the optimal duration of aspirin and clopidogrel administration after complex PCI defined as an intervention that satisfies any of the following: three vessels treated, three or more stents implanted, three or more lesions treated, bifurcation with two stents implanted, total stent length >60 mm, or CTO.27 In their post hoc patient-level pooled analysis of six randomized control trials, long-term DAPT (≥12 months) yielded significant reductions in MACE (defined as the composite of cardiac death, MI, or stent thrombosis) compared with short-term DAPT (3 or 6 months) in the complex PCI group, (adjusted HR 0.56; 95% CI [0.35–0.89]) without an increase of major bleeding at adjusted analyses. However, long-term DAPT did not improve clinical outcomes in CTO patients. Similarly, in a retrospective analysis dedicated to CTO interventions by Lee et al., ≤12-month administration of aspirin plus clopidogrel was similar to >12-month administration with respect to major adverse cardiac and cerebrovascular events (MACCE; 19.4% versus 18.8%; p=0.88; Figure 2).28 In addition, moderate or severe bleeding according to BARC criteria (type 2, 3, or 5) was also similar between the ≤12-month and >12-month group (2.5% versus 1.9%; p=0.99).
A substantial percentage of patients, estimated to be up to 30%, has resistance to clopidogrel. Multiple intrinsic and extrinsic mechanisms such as drug–drug interactions involving cytochrome P450 3A4, polymorphisms of the P2Y12 receptor and increased release of adenosine diphosphate (ADP) have been implicated.29 Routine platelet function testing to adjust antiplatelet therapy is not recommended according to current guidelines. However, high platelet reactivity (HPR) defined as ADP test ≥70% in patients with CTO lesions has been associated with lower survival. In a study by De Gregorio et al., which included 1,101 patients who underwent a CTO PCI attempt, HPR was found in 18% of the patients.30 Means for the ADP test by light transmission aggregometry were 44 ± 16% versus 77 ± 6%, respectively. Three-year survival was significantly higher in the optimal platelet reactivity (OPR) group compared with HPR patients (95.3 ± 0.8% versus 86.2 ± 2.8%; p<0.001). Interestingly, cardiac survival was similar in patients with OPR compared with those with HPR whose therapy had been escalated to either prasugrel or ticagrelor (95.3 ± 0.8% versus 90.7 ± 3.9%; p=0.172) suggesting that a tailored antiplatelet therapy in patients with high atherothrombotic burden, such as those with CTO lesions, based on platelet test, could lead to survival benefit.
The TIGER-BVS trial randomizes patients who receive CTO PCI with Absorb bioresorbable vascular scaffold (Abbott Vascular) implantation to receive ticagrelor or clopidogrel, focusing on the recovery of vascular function.31 The rationale behind this trial is that the worse CTO PCI outcomes compared with the non-CTO interventions might be partially associated with impaired vasomotor function in the vessels post PCI. Ticagrelor increases local adenosine by blocking its uptake and through its vasodilating effect can potentially improve vessel vasomotor function.32
Prasugrel or ticagrelor may be considered as an alternative to clopidogrel, in specific high-risk situations of elective stenting (e.g. suboptimal stent deployment or other procedural characteristics associated with high risk of stent thrombosis, complex left main stem, or multivessel stenting), CTO PCI (left main CTO PCI, more than one CTO PCI, CTO plus non-CTO PCI) or if DAPT cannot be used because of aspirin intolerance.33 CTOs frequently have a high burden of calcific deposit predisposing to stent underexpansion. In such cases the selection of prasugrel or ticagrelor over clopidogrel is reasonable.
Wang et al. compared two different doses of ticagrelor (180 mg loading dose, 90 mg twice daily thereafter and 120-mg loading dose, 60 mg twice daily thereafter) with clopidogrel (300-mg loading dose, 75 mg daily thereafter) in East Asian patients with CTO undergoing PCI.34 At 1-year follow-up, both ticagrelor doses reduced the rate of MACCE (6.4% versus 7.3% versus 14.2%; p=0.023 for the ticagrelor 90 mg, ticagrelor 60 mg and clopidogrel 75 mg group respectively) and target vessel revascularization (2.3% versus 2.8% versus 7.4%, respectively; p=0.047). This was at the expense of higher major bleeding (4.1% versus 0.6% versus 0.6%, respectively; p=0.016) and minor bleeding (23.4% versus 12.4% versus 11.9%, respectively; p=0.004) with 90 mg of ticagrelor, leading the authors to conclude that in East Asian patients with CTO undergoing PCI, 60 mg ticagrelor was as effective as 90 mg and at the same time significantly reduced risk of bleeding. Nevertheless, the patients included in the study were exclusively Asian and the results cannot be extrapolated to other populations. The reduction in MACCE should not be attributed exclusively to the effect of the more potent antiplatelet on CTOs but also to the more effective prevention of thrombotic events triggered by different unstable atheromatous plaques found in patients’ coronary trees.
Conclusion
Revascularization of CTO lesions is one of the most complex subsets of coronary interventions, posing often significant challenges to the interventional cardiologist. Choosing the optimum periprocedural anticoagulation and antiplatelet agent as well as long term DAPT regimen are of paramount importance. Although emerging data support that bivalirudin can be an effective and safe anticoagulant for CTO interventions, heparin is still the preferred option given the fact that its action can be reversed by protamine administration. Glycoprotein IIb/IIIa receptor inhibitors should be avoided in general, as they have been associated with increased mortality and they can increase bleeding due to an unrecognized minor perforation. Aspirin in combination with clopidogrel for 6–12 months is the standard DAPT in patients who have undergone CTO PCI. The duration of DAPT is individualized after balancing the ischemic and bleeding risk. Finally, in patients with stent underexpansion, left main CTO PCI and more than one CTO PCIs or CTO PCI plus non-CTO PCI during the same procedure, the administration of ticagrelor or prasugrel instead of clopidogrel seems reasonable.