The prevalence of tricuspid regurgitation (TR) increases with age, with up to 6% of individuals having moderate or greater TR over the age of 75, representing an ever-growing cohort.1,2 TR is known to adversely impact both quality of life and long-term survival, particularly in people with mitral regurgitation.1,3,4 Accordingly, an expanding population will stand to benefit from the development of dedicated transcatheter tricuspid valve intervention (TTVI) approaches to reduce morbidity and mortality.1,3
Mechanistically, TR mirrors mitral regurgitation (MR). Primary TR denotes abnormalities of the leaflet, chordae and papillary muscles, while secondary TR involves right ventricle (RV) dilation leading to leaflet tethering or annular dilation, and is often related to left-sided cardiac issues. Finally, isolated TR is a separate entity resulting from right atrium (RA) and tricuspid valve (TV) annulus dilation without left-sided heart disease and often presents in elderly patients with atrial fibrillation, akin to atrial MR as previously described.5–7 These differing mechanisms lend themselves to different interventional approaches.
TR quantification has proven considerably more challenging than MR quantification, with traditional scoring methods inadequately describing the magnitudes of regurgitation often encountered – an important consideration when standardizing intervention assessment. Accordingly, there is momentum to revise the TR quantification scale to better reflect the pathologies encountered by including the terms ‘massive’ and ‘torrential’, with specific criteria already proposed.8
Historically, therapeutic options for TR were limited, with guidelines supporting surgical intervention for even mild TR when present at the time of concomitant left-sided cardiac surgery.9 Interestingly, isolated TV surgery remains rare and continues to have a high mortality rate of 8.8% – the highest risk of all surgical valve interventions.10,11 The noted morbidity and mortality of TR coupled with a lack of therapeutic approaches spurred rapid innovation with numerous approaches to TTVI as a result.
To ensure standardized assessment of this rapidly evolving field, the International Multisite Transcatheter Tricuspid Valve Therapies (TriValve Registry; NCT03416166) was recently established, enabling swift outcome monitoring regardless of device type.12 Early results suggest that TTVI has better survival rates and fewer admissions for heart failure (HF) than medical therapy for symptomatic TR.13,14 Reassuringly, even in patients with massive and torrential TR, who are known to have greater mortality and HF admissions than severe those with TR, studies suggest consistent benefit with improved outcomes following successful TTVI.15,16
Enhanced awareness coupled with considerable advancements in transcatheter technology have generated a marked expansion in the TTVI space, with numerous strategies approaching this pathologic state from different angles.11
Edge-to-edge Repair
Transcatheter edge-to-edge valve repair, modeled after the surgical Alfieri repair, was firmly established as a viable therapeutic approach for valve repair in patients with MR. This concept was then extended to the TV. In some instances, combined MV and TV clips are implanted in the same sitting, yielding promising results, although this remains an area of ongoing investigation.17,18 Nonetheless, using MV devices in the TV demonstrated the feasibility of edge-to-edge repair, laying the groundwork for dedicated TV devices, which continue to improve procedural efficiency and success.
However, the TV presents unique challenges for edge-to-edge repair that do not arise in the MV. First, the TV has a complex, nonplanar anatomy with a flattened oval configuration that alters in shape and size depending on the cardiac cycle, loading conditions and pathology.11 Indeed, this anatomy often results in substantial coaptation gaps coupled with tethered leaflets that render grasping to facilitate leaflet approximation challenging.19
Novel techniques including modified steering, grasp optimization, and 'zipping' the valve together (done by starting the intervention at the annular aspect and moving centrally) have proven helpful to achieving successful reductions within challenging anatomies.20,21
In addition, TV imaging is considerably more challenging than MV imaging. While transesophageal echocardiography (TEE) remains the foundation of TV imaging, it is often limited by the TV’s complex anatomy and its anterior position, distant from the TEE probe. Acoustic shadowing of TV anatomic structures during TEE imaging can be encountered in a variety of circumstances, including in the presence of a horizontal heart axis, a lipomatous atrial septum, prosthetic mitral and aortic valves, and device leads.
The advent of multimodality imaging, including the combination of fluoroscopy, 2D and 3D TEE, intracardiac echocardiography (ICE), and cardiac CT, have provided added benefit to TV procedural planning and execution.22 For edge-to-edge TV repair, we favor a complementary strategy that integrates TEE and ICE, alternating between the modalities intraprocedurally to achieve the necessary views to enable successful edge-to-edge repair.23
ICE will continue to evolve with upcoming 3D and mapping iterations that will further optimize imaging for TV interventions.24 While imaging modalities continue to evolve, so too do the devices designed for edge-to-edge repair of the TV (Table 1).
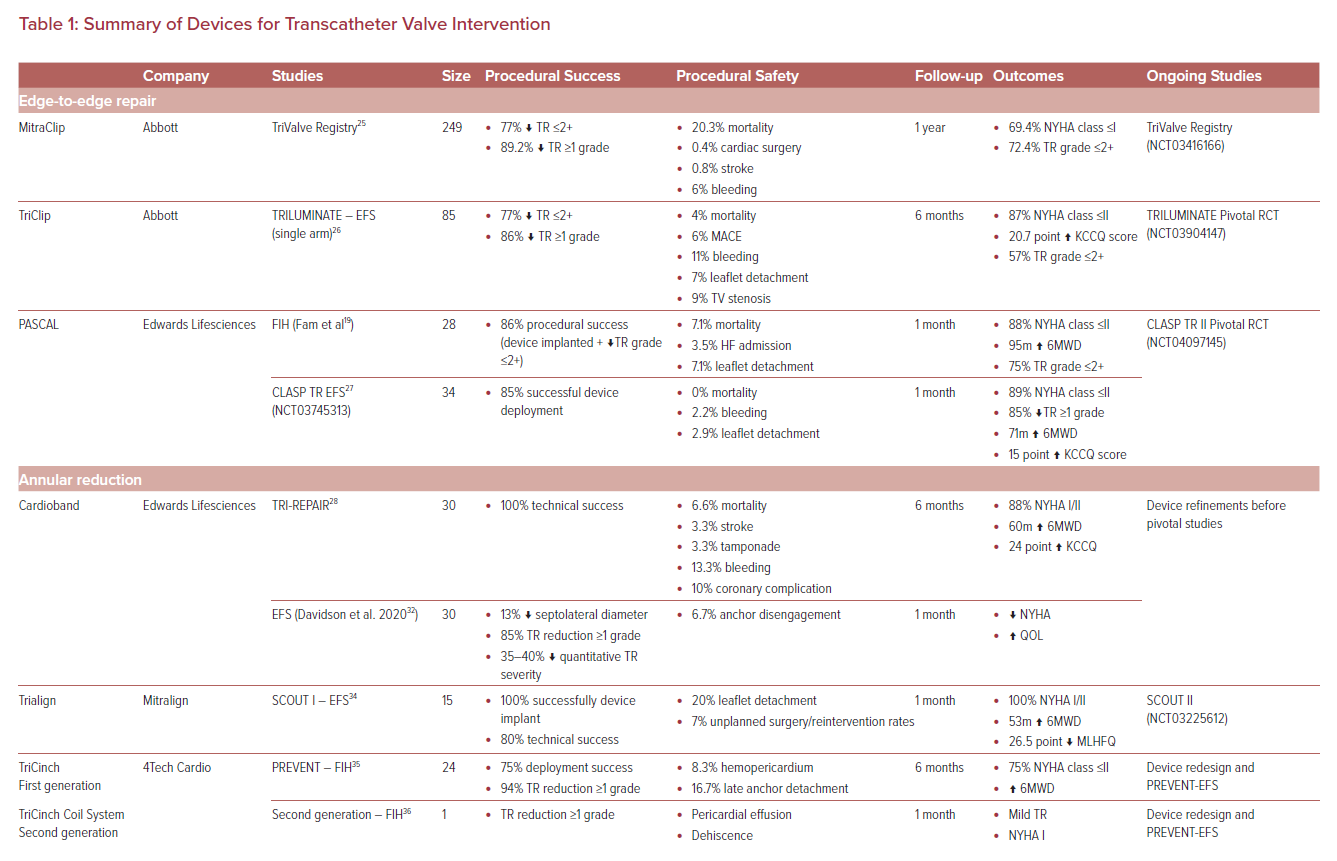
MitraClip in the Tricuspid Position and TriClip
The groundwork for TV edge-to-edge repair was laid by the early use of the MitraClip (Abbott) in the tricuspid position using modified steering approaches.21 Early compassionate use demonstrated successful deployment of the MitraClip in the TV position in 97% of cases, reducing the TR grade by one or more in 91% of patients, with sustained TR reductions up to moderate grade in 86% at 30 days.17 This was further supported to less than TriValve registry demonstrating successful reduction to TR grade up to 2+ in 77%, with long-term follow-up demonstrating excellent clinical and technical success (Table 1).25 While concomitant TV procedures performed at the time of MV interventions have acceptable safety, feasibility and learning curve profiles, dedicated studies are needed to identify the optimal approach in this cohort.18
The success of MitraClip in the TV position led to the development of the dedicated TriClip device (Abbott), which employed the same clip but with altered delivery system mechanics to optimize implantation in the TV.26 First studied in 85 patients as part of the TRILUMINATE single-arm trial, this device demonstrated 86% successful TR reduction by one grade or more, with 6-month follow-up showing sustained rates of major adverse cardiovascular events and mortality (Table 1).26 Currently, the TRILUMINATE pivotal randomized control trial (RCT) (NCT03904147) is under way to firmly establish the utility of dedicated TriClip-mediated edge-to-edge repair compared to conservative therapy.
PASCAL
The PASCAL repair system (Edwards Lifesciences) was initially developed for MR repair then extended for use in TR. The PASCAL implant, similar in design to the MitraClip, is composed of a central spacer with two adjacent paddles upon which clasps are deployed to secure the leaflets in a grasping position, offering a low-profile design to improve subvalvular manipulation (Figure 1).19
The first-in-human (FIH) compassionate patient series demonstrated a favorable procedural success rate of 86% with a 7% leaflet detachment rate. In follow-up, this translated to sustained reductions in TR in 75% of patients with improvements in New York Heart Association (NYHA) class and 6-minute walking distance (6MWD; Table 1).19
The CLASP TR EFS (NCT03745313) enrolled 34 patients (29 patients implanted) at multiple centers in the US and demonstrated that 85% of implanted patients achieved at least 1 grade of TR reduction, with 52% having moderate or less TR after the procedure.27 Major adverse event rates at 30 days were 5.9% and improvements in NYHA class, 6MWD and quality of life were all observed at 30 days.27 The CLASP TR II Pivotal Trial (NCT04097145) is randomizing patients to device or optimal medical therapy and will provide definitive evidence regarding the role of edge-to-edge repair for TR.
Annular Reduction
Annular dilation plays a major role in many TR pathologies, with numerous surgical approaches focusing on annuloplasty as a primary approach, particularly in the early stages of TR.11 Accordingly, percutaneous annular reduction approaches aim to mimic surgical annuloplasty by reducing the size of the tricuspid annulus, reducing the coaptation gap, and restoring leaflet approximation.
Challenges with this approach include achieving successful annular reduction with a secure device implant while not impinging on the adjacent conduction system, right coronary artery, or aortic or coronary sinuses.11
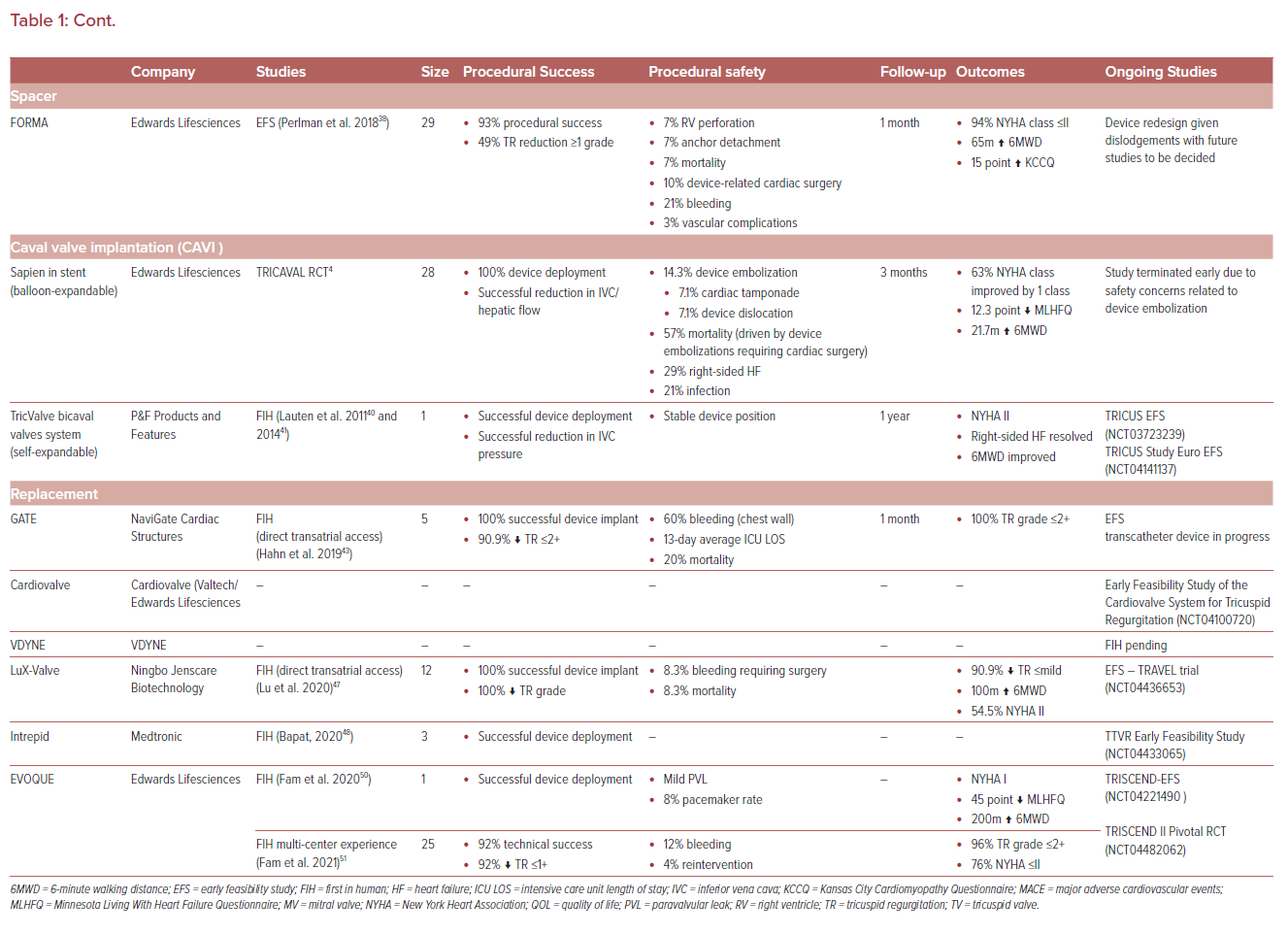
Cardioband
The Cardioband tricuspid repair system (Edwards Lifesciences) was adapted from the mitral system and uses a sutureless, adjustable band attached to the atrial aspect of the TV annulus. The band is delivered via a 24 Fr venous access sheath and secured in position by multiple anchors. Once the band is fixed in place, the size adjustment tool can then be used to adjust the annular size (Figure 2).28
This device showed promise in the TRI-REPAIR study, with sustained improvements in both clinical and echocardiographic results (TR grade) with an excellent safety profile sustained at both 1 and 2-year follow-up (Table 1).28–30 The Cardioband early feasibility study (EFS) in the US included 30 patients who underwent repair, and found a 13% reduction in septolateral diameter, and 35–40% reduction in TR severity by quantitative measures. In 85% of patients, TR decreased by one grade or more and the majority of patients experienced improvements in NYHA class and quality of life at 30 days.31,32 Ongoing device refinements are planned before pivotal studies commence.
Trialign
The Trialign system (Mitralign) was developed from the earlier Mitralign annular reduction systems. From a transjugular approach, this device achieves annular reduction by plicating the TV to create a bicuspid valve. This is achieved via radiofrequency-mediated placement of pledgets at the posteroseptal and anteroposterior commissures, which are then drawn together to, essentially, remove the posterior TV leaflet and reduce TR.33
This approach was assessed in the SCOUT I EFS trial in 15 patients, which demonstrated overall excellent procedural success but was limited by 20% leaflet detachment rates for which device refinements were pursued.34 The SCOUT II trial (NCT03225612), now ongoing, is intended to improve the understanding of the role of this therapy in TR reduction (Table 1).
TriCinch
Another approach to annular remodeling is employed by the TriCinch device (4Tech Cardio) and intended for secondary TR. This device is delivered via the femoral approach with a 24 Fr sheath; a corkscrew is fixated supra-annularly into the anteroposterior TV annulus, with an anchor line connected to a stent placed within the inferior vena cava (IVC).
The PREVENT study examined this first-generation device with successful deployment in 75% of cases with a TR reduction of one grade or more in 94%.35 However, 8% of patients experienced hemopericardium and 16.7% late detachment of the annular anchor. This led to a device redesign yielding the TriCinch Coil System (4Tech Cardio), which uses an epicardial coil with two hemostatic seals placed by creating a carbon dioxide pneumopericardium to enable visibility of the anchor placement in the pericardial space.36 This device was limited by dehiscence and hemorrhagic pericardial effusions (Table 1).35 Ongoing EFS work on this technology is forthcoming and the role of this therapy remains to be defined.
Spacer
FORMA
Physical filling of the coaptation defect is an alternative strategy pursued in so-called spacer therapies; rather than approximating the surrounding tissue to fill the defect, they physically fill the space themselves.
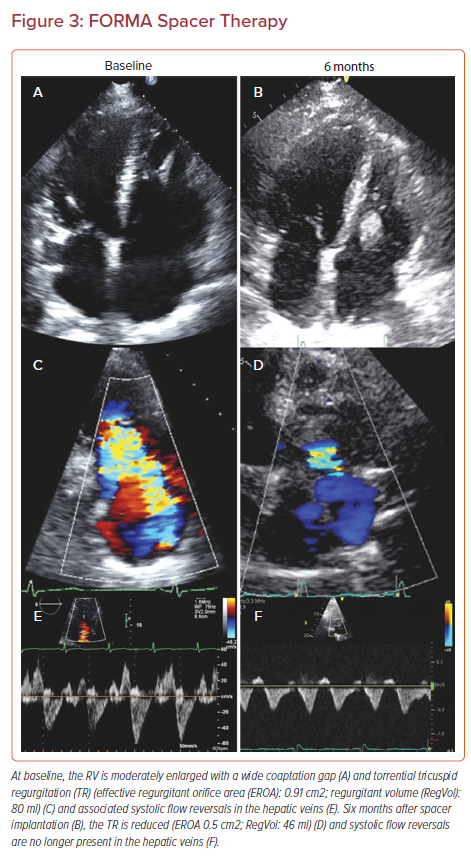
The FORMA device (Edwards Lifesciences) has been the primary device using this mechanism and is implanted via a 24 Fr sheath via either the left subclavian or axillary vein. The anchor is first implanted at the right ventricle (RV) apex and a foam-filled spacer balloon is then advanced and tethered to the apical rail, securing the device in position (Figure 3). Early compassionate use of the FORMA device in 18 patients yielded an 89% procedural success rate with one RV perforation and one device-related thrombus; however, it provided 79% of patients with improvement to NYHA I/II and sustained reduction to less than moderate TR in 46%.37 The subsequent EFS in 29 patients yielded promising results as well, with a good efficacy profile, but RV perforation and late anchor dislodgement challenged this technology and prompted a redesign (Table 1).38 Accordingly, the future of the FORMA device is uncertain at present.
Caval Valve Implantation
A unique, outside-the-box strategy for dealing with the challenges of the TV was to move the device therapy outside the TV, with so-called heterotopic valve implantations. This strategy uses traditional valve replacement devices but deploys the valves in the caval vessels adjacent to the right atrium to mitigate the effects of TR on surrounding organs while not sparing the RA. This caval valve implantation (CAVI) strategy has been approached with both balloon-expandable and self-expanding valves.
The TRICAVAL RCT explored the use of a standard balloon-expandable Sapien valve (Edwards Lifesciences) implanted within a stent deployed within the IVC.4 This strategy raised significant safety concerns related to valve dislocations and procedural complications requiring surgical interventions, which prompted an early stop to the study for safety reasons.4
An alternative strategy using a dedicated, self-expanding nitinol TricValve (P&F Products and Features) placed within the superior and/or inferior vena cava offered another approach, eliminating the need for pre-stenting and mitigating the radial forces applied to the caval structures with balloon-expandable valves.39 Early FIH work with this technology appeared promising, with symptomatic improvement and a favorable safety profile (Table 1).40,41 The TRICUS study (NCT03723239) and TRICUS study Euro (NCT04141137) are enrolling to achieve CE mark status for the TricValve device.
However, the CAVI strategy, if successful, would likely fill a niche role in those with end-stage right-sided HF experiencing significant annular dilation that precludes the use of other devices. In these circumstances, the effects of RA ventricularization and persistent overload remain to be explored. Taken together, while intriguing, it is unlikely that this approach will represent the primary modality of TTVI going forward.
Replacement
Transcatheter tricuspid valve replacement (TTVR) is challenged by the anatomic complexities of the TV and annulus, which are often further deviated in the presence of significant TR. Indeed, the large annulus and lack of a supportive matrix for valve anchoring pose significant limitations for TTVR implantation. A single early report of TTVR in a native TV required pre-stenting the TV annulus with covered stents followed by implantation of two Sapien valves for positioning and was complicated by wire-induced pulmonary artery perforation, highlighting the significant challenges the TV presents for TTVR.42 However, dedicated valves designed for the mitral and tricuspid positions are now showing promise in ongoing investigations.
GATE
The GATE bioprosthetic device (NaviGate Cardiac Structures) was one of the first TTVR devices available, although is was developed initially as a surgical implant. This device is advanced via a mini-thoracotomy approach using a 42 Fr introducer sheath to enable placement of the conical nitinol stent, which encompasses three pericardial leaflets that are secured in place by anchoring RA winglets and RV tines.43
Its use was first demonstrated in two patients, one of whom had an existing TV annuloplasty ring.44 The complete compassionate FIH experience demonstrated successful valve implantation in all patients with successful TR reduction to a grade up to 2+ in all patients, with sustained reduction in TR severity at 30-day follow-up.43 However, the direct atrial access resulted in significant bleeding complications and prolonged intensive care unit admissions, with a 20% mortality rate in the FIH experience (Table 1).43 Upcoming device iterations are expected to enable transjugular access, which will likely yield marked improvements in outcomes. Early feasibility studies are now under way to establish the safety and efficacy of this device.
Cardiovalve
The Cardiovalve (Cardiovalve) is a percutaneously delivered, low-profile (15 mm height), double-framed valve with tri-leaflet bovine pericardial leaflets. This valve showed early promise in FIH studies following MV implantation via a transseptal approach.45 Cardiovalve has now gained Food and Drug Administration (FDA) approval to proceed with an EFS study for TV implantation with enrollment expected shortly (NCT04100720).
VDYNE
The VDYNE valve (VDYNE) affords a unique approach based upon a 30 mm porcine inner valve encased in an outer annular ring; the outer rings offered vary in size to provide five valve sizes, providing an anatomic fit to the native annulus. The device is deployed through a single 28 Fr transfemoral sheath, employing a novel side delivery system, abrogating the need for hardware in the RV while allowing the operator to reposition and recapture the device. The device uniquely offers a pop-off mechanism in the event of afterload mismatch. The VDYNE valve will soon be undergoing FIH assessments.46
LuX-Valve
The LuX-Valve (Ningbo Jenscare Biotechnology) is a radial-force independent system with four components: a bovine pericardial valve; a self-expanding nitinol valve stent with an atrial disc; an interventricular septal anchor tab; and two graspers. This device is delivered via right thoracotomy with a 32 Fr system. The FIH study demonstrated promising results with 100% procedural success and no more than mild residual TR in 90.9% of patients.47 Ongoing assessment is being performed in a dedicated EFS study (TRAVEL trial; NCT04436653).
Intrepid
The Intrepid valve (Medtronic) was originally developed for transcatheter mitral valve repair, but adapted for the TV position. It provides three annular sizes, built around a single 29 mm bovine valve that is delivered via transfemoral access, and has yielded promising FIH results and safety.48 This device received FDA breakthrough status in 2020 and is now actively enrolling in an EFS (NCT04433065).49
EVOQUE
Similarly, EVOQUE (Edwards Lifesciences) was initially developed for the MV position with considerable refinements employed to enable its use for the TV. This system enables 44 mm, 48 mm and 52 mm valves to be implanted via a 28 Fr delivery system.
The FIH implant was recently performed, with successful implantation being achieved with mild PVL. Follow-up yielded clinical improvement to NYHA class I, 6MWD increased by 200 meters and a 45-point improvement in Minnesota Living with Heart Failure Questionnaire score. Echocardiographic follow-up showed stable mild paravalvular leak (PVL) and a mean gradient of 2 mmHg with reduced RV volumes (Table 1).50 The subsequent multicenter FIH experience was published shortly after; this included 25 patients with 92% technical success, and durable improvements in clinical and echocardiographic parameters at 30 days.51 Accordingly, the TRISCEND EFS (NCT04221490) and TRISCEND II Pivotal RCT (NCT04482062) are actively enrolling to establish this therapy as a viable TTVR approach.
Remaining Questions
Several important questions will be answered by ongoing device trials in the TTVI field, including whether reduction of TR leads to improved survival and other endpoints including hospitalizations, diuretic use and quality of life as compared to medical therapy.
No randomized studies to date, including both surgical and percutaneous approaches, have shown a survival benefit of TR treatment. Hence, the next phase of studies are critically important to establish our fundamental knowledge of this pathology while discerning the optimal population and patient anatomy that stand to benefit most from the specific therapies studied to improve clinical outcomes.
The durability of the various TR therapies will need to be carefully studied to understand their role and potential application in a lower-risk population. Optimal strategies for the management of TR in combination with other valvular disease will also require dedicated evaluation.
Conclusion
The rising awareness of the importance of TR coupled with the rapid evolution of transcatheter technologies have yielded marked advances in TTVI development. While the TV poses many unique challenges, the breadth of technological approaches to this pathologic state remain diverse, with the future TTVI armamentarium likely to include multiple modalities with dedicated devices for specific pathology subtypes. While considerable work remains, TTVI, especially TTVR, are poised to rapidly become the standard therapeutic strategies for TR.