Over the past decade, transradial access has grown in popularity. With percutaneous coronary intervention success rates similar to transfemoral access, transradial coronary angiography is associated with fewer vascular and bleeding complications, earlier ambulation, greater postprocedural comfort, and better cost-effectiveness.1–5 A meta-analysis by Ferrante et al. showed a 77% reduction in major vascular complication by using transradial access (OR 0.23; 95% CI [0.16–0.35]).6 In addition, transradial access also reduces the incidence of death, MI, stroke, and overall mortality in ST-elevation MI patients.1 Therefore, the ‘radial-first’ strategy was recommended in the 2015 European Society of Cardiology guidelines for the management of acute coronary syndrome as a class I indication.7
Transradial access has intrinsic challenges and a steep learning curve. Its success rate is highly dependent on operator experience. Fluoroscopic time, radiation exposure, procedural success, access site crossover, and access site complications can be minimized by operator experience and proficiency.8,9 Transradial access-related vascular complications of coronary intervention requiring surgical or percutaneous intervention are reported at 0.3%.10 The need for surgical intervention can be decreased by prompt recognition of complications by operators and cardiac catheterization laboratory team members.11 This article reviews vascular complications of transradial cardiac catheterization and their management to promote awareness, limit the incidence of complications, and encourage timely intervention when complications do occur (Table 1).
Radial Artery Spasm
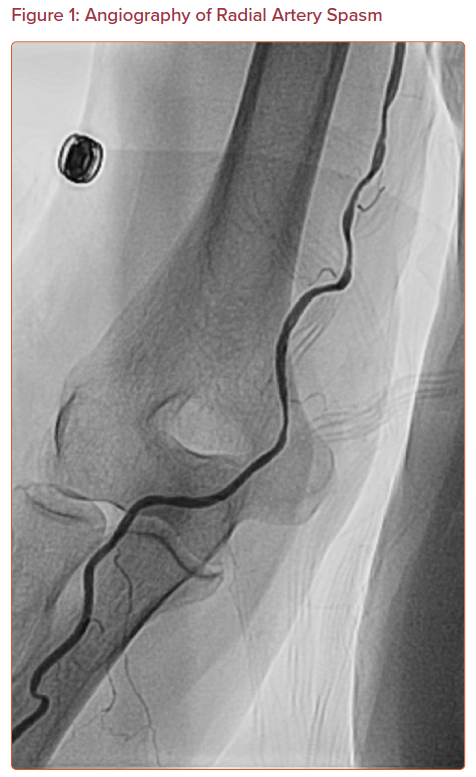
Radial artery spasm is recognized when catheters initially pass through the arm, but subsequent manipulation meets with resistance and causes pain in the arm (Figure 1). This is a common complication and the most common cause of transradial access site crossover.12,13 The incidence of radial artery spasm is 4–5% in large randomized control trials (RADIAL and RIVAL trials).1,14–16 Younger age, female sex, diabetes, and lower BMI are independent predictors of radial artery spasm, as are small radial artery diameter, large sheath:artery size ratio, and multiple catheter exchanges.12
Most radial artery spasm is mild, and multiple techniques can be used to prevent and treat it.16 Adequate pain control and sedation are the key to prevent radial artery spasm. Deftereos et al. reported that patients with adequate sedation and analgesia had a lower incidence of radial artery spasm compared with patients without adequate sedation (8.3% versus 2.6%; p<0.001) and less frequent access site crossover (15% versus 9.9%; p=0.001).17 Although not common, general anesthesia or regional nerve block is an alternative option for adequate pain control.
Appropriate equipment selection can minimize the occurrence of radial artery spasm. Long radial sheaths with a tapered dilator and hydrophilic coating are associated with lower rates of radial artery spasm.12,18 Use of a ‘universal’ catheter designed to access both the left main ostium and the right coronary artery ostium (e.g. Terumo Tiger and Jacky catheter) reduces catheter exchanges, which reduces radial spasm.19
Antispasmolytics or vasodilators, such as calcium channel blockers (intra-arterial verapamil or diltiazem) and nitroglycerine, are commonly injected into the radial artery after access is obtained, and some operators repeat it after every catheter exchange.20 While many studies have evaluated the efficacy of different agents, there is no consensus on a standard regimen to prevent radial artery spasm. A systematic review by Kwok et al. proposed that intra-arterial verapamil 5 mg alone or in combination with 100–200 µg of nitroglycerine is the most effective antispasmolytic.15
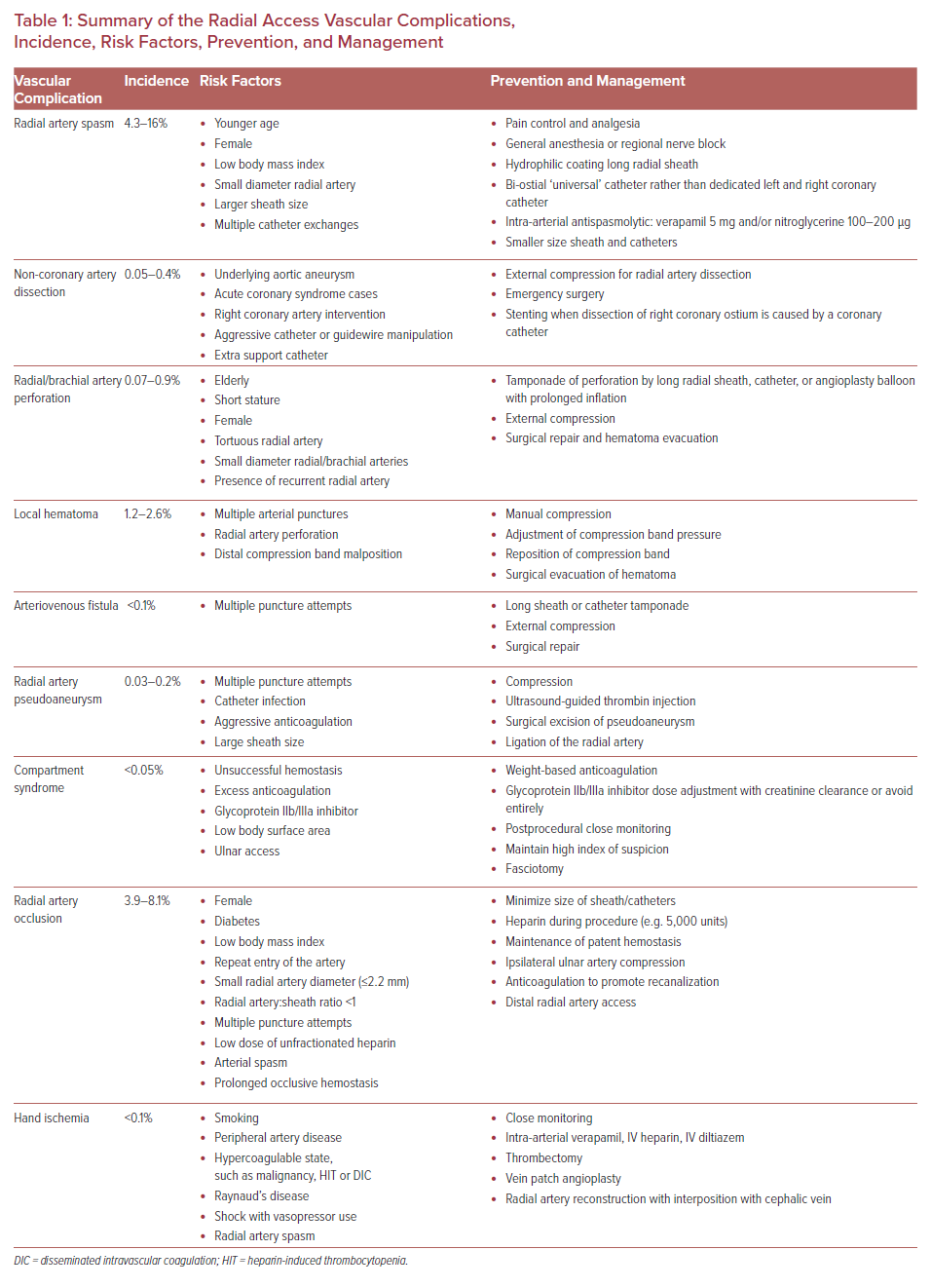
Non-coronary Artery Dissection
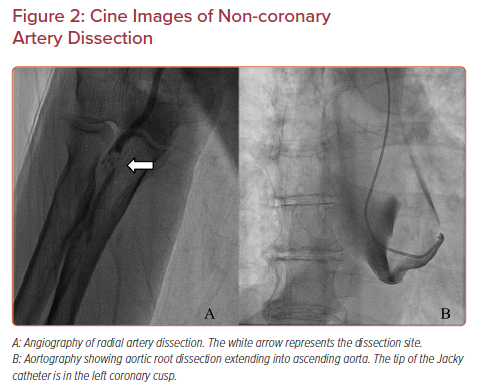
A tear in the wall of a major non-coronary artery leading to the collection of blood within the layers of arterial wall is a rare complication of transradial cardiac catheterization with an incidence of 0.02–0.4%.11,12 The incidence is higher in acute coronary syndrome cases than in elective cases, and it is also higher in percutaneous coronary intervention than in diagnostic cardiac catheterization.21 Arterial dissection can be prevented by ensuring that the catheter is not passing through a radial loop or recurrent radial artery; if there is resistance to initial passage of the catheter, then angiography should be undertaken to ensure that the artery is large enough and straight enough to accommodate the catheter. Using a smaller guide within the diagnostic guide or balloon-assisted tracking with an angioplasty balloon can prevent dissection of tortuous radial or brachial arteries. The most common dissection site is the radial artery (Figure 2A).
Catheter dissections of the radial and brachial artery can usually be managed conservatively by proceeding with the procedure. The catheter itself usually seals the dissection. Arteriography at the conclusion of the procedure is advisable to document the final status of the dissected artery.
Iatrogenic ascending aortic dissection is a serious complication that can occur with either radial or femoral access. It is an immediate threat to life when it causes hemodynamic instability. In the majority of case reports, the ascending aortic dissection is caused by right coronary artery intervention causing retrograde dissection to the aorta.22 The risk of dissection is increased by aggressive catheter or guidewire manipulation, and by use of extra backup catheters.21
Occasionally, with transradial access, innominate tortuosity can render catheter passage difficult, leading to innominate dissection that extends into the ascending aorta. This occurred in our catheterization laboratory when a 66-year-old woman with a 5.2 × 5.2 cm ascending aortic aneurysm and severe aortic regurgitation underwent diagnostic transradial cardiac catheterization. Upon introduction of a Jacky diagnostic catheter over a 0.035 inch Terumo Glidewire into the ascending aorta, the patient complained of chest pain radiating to her neck. Cine images showed a large ascending aortic dissection involving the aortic root and proximal arch (Figure 2B). CT imaging showed that it extended into (and presumably started at) the innominate artery. The patient underwent emergent Bentall root replacement, aortic valve replacement, and coronary artery bypass of all coronary arteries with ligation of the native left main artery, and was discharged 1 month later.
Radial Artery Perforation
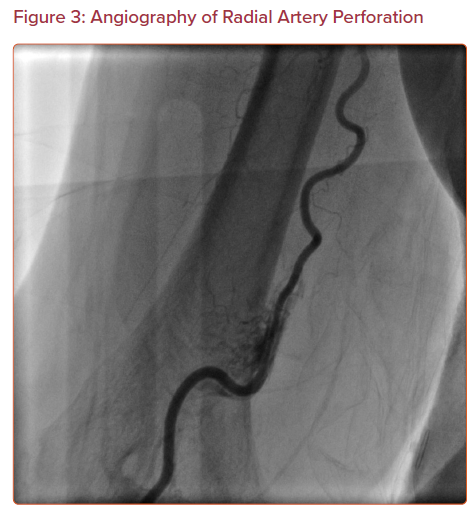
Perforation of the arterial wall with blood extravasation may occur in the radial or brachial artery (Figure 3). It may also present as a hematoma formation proximal to the access site in the forearm. This is an uncommon complication, with an incidence of 0.07–0.9%.11,23,24 Although radial artery perforation is rare, it can lead to severe forearm hematoma and compartment syndrome if it is not managed promptly.
Radial artery perforation occurs most often in patients who are elderly, short, female, or have tortuous arteries. Many cases of perforation occur when a guidewire passes up a small recurrent radial artery that is too small to accept the catheter. Occasionally, a diagnostic catheter will pass, but when the diagnostic procedure is converted to an intervention, the guide catheter will be too large for the recurrent radial artery, and an operator who pushes the catheter too hard can easily cause dissection and perforation. In any situation where resistance to catheter passage is noted, angiography is indicated to define the anatomy.
Intraprocedural recognition of radial artery perforation does not prohibit the continuation of the procedure if the wire can be passed proximally into the innominate artery, because the intraluminal long radial sheath or catheter can tamponade the perforated segment.24 At the end of the procedure, radial artery angiography should be obtained to document that the perforation has sealed. Prolonged inflation of a peripheral or coronary artery balloon can be used to seal the perforation if a persistent leak through the perforation is noted.25 External compression with an elastic bandage or blood pressure cuff inflation at the level of systolic blood pressure may also be utilized.26 A delay in making the diagnosis or inability to achieve hemostasis by conservative measures may lead to the need for surgical repair of the arterial perforation, evacuation of hematoma, and treatment of compartment syndrome if necessary.
Hematoma
Hematoma is a local accumulation of blood within the soft tissues causing a swelling. It can extend proximally from the access site to the forearm and arm. Femoral access site bleeding is associated with increased mortality.27 Clinically significant transradial access-associated local hematoma incidence is 1.2–2.6%, which is 60% lower than in transfemoral access.1,10,12,28–30 However, in the radial and ulnar cohorts in the SPIRIT of ARTEMIS study, the overall rate of local hematoma after transradial diagnostic coronary angiography was 23% when postprocedural vascular ultrasonography was performed on every patient, although most of these were not clinically significant.16 Most hematomas in this cohort were <5 cm in size. In this study, there was no difference in the incidence of hematoma between standard-dose (50 IU/kg bodyweight) and high-dose (100 IU/kg bodyweight) intra-procedural unfractionated heparin.
The development of hematoma depends on the experience of the operator and cardiac catheterization laboratory team members. Multiple arterial puncture attempts, inadequately managed radial artery perforation, and misplacement of a radial artery compression device (TR Band or HemoBand) are associated with hematoma. Early recognition and treatment of local hematoma are crucial to prevent serious complications, such as compartment syndrome.
When a hematoma is noted, manual compression, adjustment of the compression band pressure, or repositioning of the compression band may be sufficient. However, if the hematoma progresses proximally in the forearm, and particularly if the forearm becomes tense and swollen, or if the patient complains of anesthesia or pain in the hand, compartment syndrome should be suspected, and emergent vascular surgical consultation should be obtained.
Arteriovenous Fistula
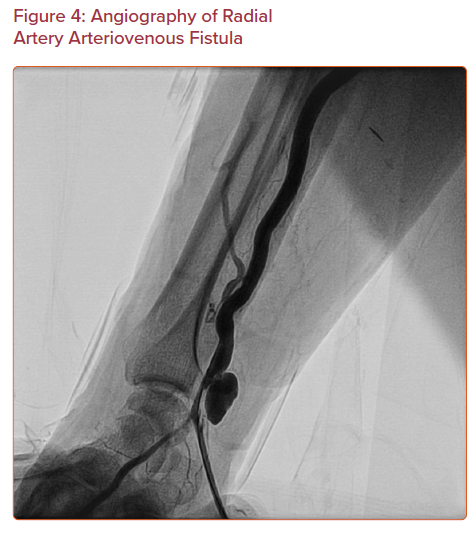
Arteriovenous fistula is an abnormal connection between an artery and a vein (Figure 4). This is a rare complication with an incidence less than 1 in 1,500. Only 12 isolated cases out of 17,870 patients are reported in three different cohorts.11,16,23 It may be asymptomatic, or it may present with persistent pain and swelling at the puncture site with visibly dilated veins and a palpable thrill. Multiple punctures increase the risk of arteriovenous fistula, because the radial artery is surrounded by small veins.
Arteriovenous fistula can be managed conservatively, similar to radial side branch perforation, if it is recognized during procedure. If a J-tip wire can pass through the artery proximally, then place the long sheath or catheter across the damaged segment. The sheath or the catheter will seal the connection between the artery and the vein. Prolonged compressive bandage can be used as an adjunctive treatment. Indications for surgical repair are symptomatic arterial steal phenomenon, venous congestion of extremity, or high-output cardiac state.30
Radial Artery Pseudoaneurysm
Pseudoaneurysm results from a penetrating injury of the arterial wall (e.g. catheter access to the artery) causing a pulsating extraluminal hematoma and contained hemorrhage that communicates with the arterial lumen. The usual presentation is a pulsatile mass with or without tenderness several days after the catheterization procedure. The diagnosis is confirmed by a systolic murmur and Doppler ultrasound showing to-and-fro flow from the radial artery. The incidence of radial artery pseudoaneurysm due to transradial access is 0.03–0.2%.1,16,23 Risk factors of pseudoaneurysm are multiple puncture attempts, catheter infection, aggressive anticoagulation therapy, and large sheath sizes.31
Literature on radial artery pseudoaneurysm management is limited. Treatment is occlusive compression using a pneumatic radial artery compression band applied against the radial artery proximal to pseudoaneurysm for 3–4 hours followed by semi-occlusive compression using an elastic bandage for 24 hours.32 If this fails, ultrasound-guided thrombin injection can be used, with the caveat that any thrombin escaping the pseudoaneurysm can cause distal limb ischemia. Indications for surgical excision of a pseudoaneurysm are pain, diameter >3 cm, and failure of more conservative treatments.33
Compartment Syndrome
Compartment syndrome is due to compression of nerves, veins, arteries, and muscles inside the forearm compartment, leading to ischemic necrosis of the tissue. Its sign is well known as the ‘five Ps,’ including pain, pallor, paresthesias, pulselessness, and paralysis. The incidence is reported at 0.004–0.05%, none of the 3,507 patients in the transradial access group in the RIVAL trial developed compartment syndrome.1,16,34 Risk factors for compartment syndrome include radial artery perforation, unsuccessful compression at the puncture site, excess anticoagulation, glycoprotein IIb/IIIa inhibitor administration, and low body mass index.11 When ulnar access is used, entry of the artery more than 2 cm proximal to the wrist can lead to compartment syndrome, because the artery is deep and not easily compressed against underlying bone.
Prevention of compartment syndrome is critical, because its management is difficult. A high index of suspicion, early recognition, and urgent management of hematoma are of the utmost importance to prevent compartment syndrome and the need for fasciotomy. A specific postprocedural monitoring protocol should be established to ensure timely response and proper management to minor vascular complications before they evolve into compartment syndrome. Doses of anticoagulation and glycoprotein IIb/IIIa inhibitor during the procedure need to be adjusted for weight and creatinine clearance. If compartment syndrome is diagnosed, emergent vascular surgery consultation followed by fasciotomy is needed to prevent long-term consequences, such as limb loss or Volkmann contracture after fasciotomy.34
Radial Artery Occlusion
Radial artery occlusion is due to thrombus formation secondary to intimal injury. Radial artery occlusion is a benign condition and most patients who have intact collaterals from the ulnar artery are asymptomatic, because palmar circulations remain protected. The concern for interventional cardiologists is that radial artery occlusion precludes repeated intervention through the same access site. Sinha et al. found that radial artery occlusion was identified by color Doppler ultrasound in 17.4% of their patients on the first day after the transradial catheterization.3 However, 60–70% of these occluded cases later have spontaneous recanalization of their radial artery.35,36 At 6 months postprocedure, radial artery occlusion diagnosed by Doppler was 5.1%; this incidence is consistent with other studies that report radial artery occlusion in 3.9–8.1% of patients8,14,16,35 Nonetheless, only 0.2% of radial access patients developed symptomatic radial occlusion needing medical attention.1 The symptoms of radial artery occlusion include hand or finger pain or discoloration, weakness, cold, or sensory deficit. Because of recruitment of ulnar artery collaterals, non-invasive tests for collateral hand circulation, such as the Allen or Barbeau test, do not predict the occurrence of radial artery occlusion and should not be used for access site selection.
Predisposing factors of radial artery occlusion are female sex, diabetes, lower BMI, repeat entry of radial artery, smaller arterial diameter at baseline (2.2 mm or smaller), and radial artery:sheath ratio <1.35–38 Risk factors related to the procedure are multiple arterial puncture attempts, low unfractionated heparin dose, radial artery spasm, and prolonged occlusive hemostasis.16,23 Prolonged occlusive hemostasis is defined as prolonged compression and absent flow during postprocedural hemostasis, especially at the final assessment before radial band removal.
Various techniques have been developed to prevent radial artery occlusion. First, the SPIRIT of ARTEMIS study showed that high-dose heparin (100 IU/kg bodyweight) was associated with a lower rate of radial artery occlusion when compared with low-dose heparin (50 IU/kg bodyweight), with an OR of 0.35 (95% CI [0.22–0.55]; p<0.001). The number needed to treat with high-dose heparin to avoid one case of radial artery occlusion is 19.6.16
Second, the PROPHET Study shows that maintenance of patent hemostasis is effective in reducing the radial artery occlusion rate after transradial catheterization.39 After a radial artery compression band is secured around the wrist at the site of artery access, “patent hemostasis” is confirmed by a plethysmographic signal on pulse oximeter sensor over the index finger when the ipsilateral ulnar artery is occluded transiently. If there is no signal, the radial artery compression band should be loosened until plethysmographic signal returns or bleeding occurs. If the radial artery patency can be maintained and the hemostasis is achieved, the compression band should be left in place for 2 hours. The patency of the radial artery during compression should be checked at least every hour.37
Third, ipsilateral ulnar compression can be used for both prophylaxis and treatment of acute radial artery occlusion. For prophylaxis, occlusive ulnar artery compression is applied concomitantly with patent radial artery hemostasis.40 As multiple puncture attempts is one of the risk factors for radial artery occlusion, ultrasound guidance for transradial artery access should be used to reduce the number of forward attempts required for access and improve the first-pass success rate according to the RAUST.41 For acute radial artery occlusion that is identified by duplex ultrasound within 3–4 hours after radial artery compression band removal, immediate ulnar artery compression for 1 hour using the same compression band increases the peak velocity of blood flow into the radial artery on the repeat ultrasound assessment.42
Once radial artery occlusion is confirmed by ultrasound, another treatment option is administration of systemic anticoagulation, such as low-molecular-weight heparin for 4 weeks. Low-molecular-weight heparin treatment increases the patency rate of the radial artery up to 86% after 4 weeks of treatment.43 If vascular access is needed in patients with radial artery occlusion, distal radial artery access can be used for the catheterization.
Distal radial artery access involves cannulation of the radial artery on the dorsum of the hand where it emerges from the anatomical snuffbox. Distal radial access improves patient comfort, reduces the risk of radial artery occlusion, and also can be used as a treatment for retrograde recanalization of the occluded radial arteries.44
Patients who have intact collaterals from the ulnar artery are asymptomatic despite radial occlusion, because the palmar circulation provides collateral flow. The occurrence of hand or finger pain, or discoloration, weakness, cold, or sensory deficit should prompt immediate evaluation and, if necessary, consultation with a vascular surgeon. If the diagnosis is confirmed, then aggressive treatment is indicated, as described above.
Hand Ischemia
Hand ischemia is a rare complication of transradial access with an incidence less than one in 1,000.45 The symptoms of hand or finger ischemia are dusky appearance of the hand or finger, paresthesia, weakness, pain, and cyanosis. There is an absence of Doppler signals in the ipsilateral radial artery and palmar arch in conjunction with a compromised ulnar artery collateral flow on ultrasound. The etiology is assumed to have two concurrent events: embolization of radial artery thrombus and in situ thrombosis of collateral vessels from vasospasm.
The abnormal result of a non-invasive test for collateral hand circulation (Allen test) does not predict clinical hand ischemia sequelae after cardiac catheterization, because recruitment of ulnar artery circulation may occur acutely during catheterization and prevent hand ischemia. Only sporadic cases of hand or finger ischemia after transradial catheterization have been reported. The following risk factors were collected from case reports and extrapolated from the Valentine et al. cohort that included all patients presenting to vascular surgery after radial artery cannulation: smoking, peripheral artery disease, hypercoagulable state (e.g., malignancy, heparin-induced thrombocytopenia, disseminated intravascular coagulation), Raynaud’s disease, shock state with vasopressor use, and radial artery spasm.45
Management of hand ischemia requires close monitoring of hand circulation postprocedure. In mild cases, medical management with intra-arterial verapamil in combination with 6 hours’ treatment with IV diltiazem and low-dose IV heparin has returned palpable radial and ulnar artery pulses. Severe cases causing ischemia of the entire hand and requiring surgical intervention generally have a poor outcome and 50% require finger amputation. Surgical management includes operative thrombectomy and arterial patch angioplasty or radial artery interposition with the cephalic vein. Radial reconstruction with interposition vein graft has a better success rate than a vein patch.45
Conclusion
Transradial access is becoming the favored access route for cardiac catheterization. Invasive cardiologists and cardiac catheterization team members should be familiar with the vascular complications. Early detection and treatment is vital, as delayed diagnosis leads to worse complications and the need for surgical intervention.
Radial artery spasm is the most common cause of access site crossover. Radial spasm is prevented with adequate pain control, and treated with verapamil and/or nitroglycerine. Hemorrhagic complications, such as perforation, hematoma, arteriovenous fistula, and pseudoaneurysm, are generally treated by prolonged compression. Precise detection and management of hemorrhagic complications will prevent development of compartment syndrome, which can leave the patients with long-term morbidity after fasciotomy. Thrombotic complications, such as radial artery occlusion and hand ischemia, are treated with anticoagulation. Preventive strategies and vigilant postprocedural monitoring should be implemented in daily practice at every cardiac catheterization laboratory.